HOW TO READ THE PERIODIC TABLE THE PERIODIC
HOW TO READ THE PERIODIC TABLE
THE PERIODIC TABLE • An ELEMENT is a substance that cannot be broken down into simpler substances by chemical or physical means. • Is made of the same type of atoms. • Ex. Gold elements are made of gold atoms • Sometimes called a pure substance • Each element has their own little box on the periodic table with valuable information
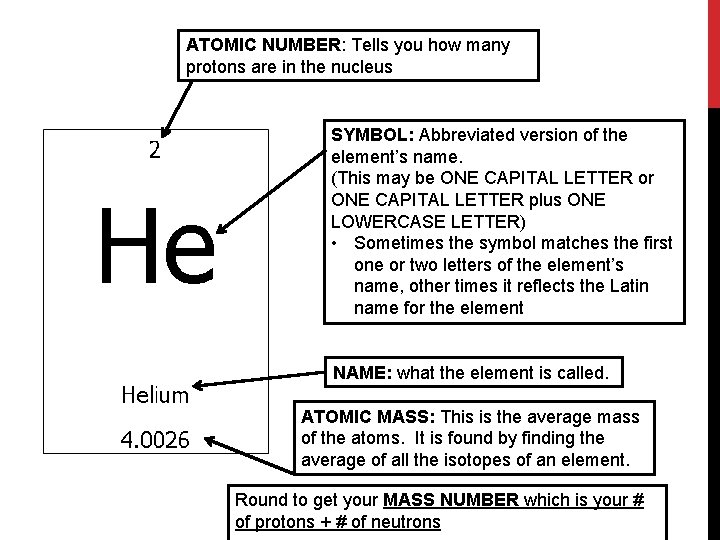
ATOMIC NUMBER: Tells you how many protons are in the nucleus SYMBOL: Abbreviated version of the element’s name. (This may be ONE CAPITAL LETTER or ONE CAPITAL LETTER plus ONE LOWERCASE LETTER) • Sometimes the symbol matches the first one or two letters of the element’s name, other times it reflects the Latin name for the element NAME: what the element is called. ATOMIC MASS: This is the average mass of the atoms. It is found by finding the average of all the isotopes of an element. Round to get your MASS NUMBER which is your # of protons + # of neutrons
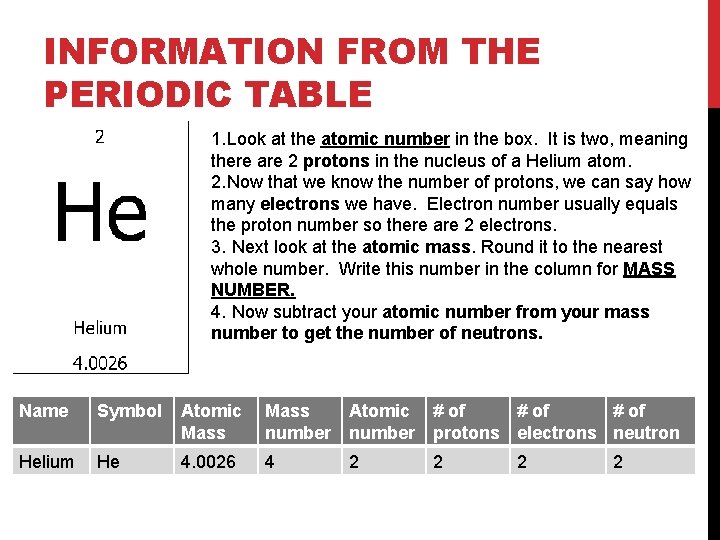
INFORMATION FROM THE PERIODIC TABLE 1. Look at the atomic number in the box. It is two, meaning there are 2 protons in the nucleus of a Helium atom. 2. Now that we know the number of protons, we can say how many electrons we have. Electron number usually equals the proton number so there are 2 electrons. 3. Next look at the atomic mass. Round it to the nearest whole number. Write this number in the column for MASS NUMBER. 4. Now subtract your atomic number from your mass number to get the number of neutrons. Name Symbol Atomic Mass number Atomic number # of protons electrons neutron Helium He 4. 0026 4 2 2
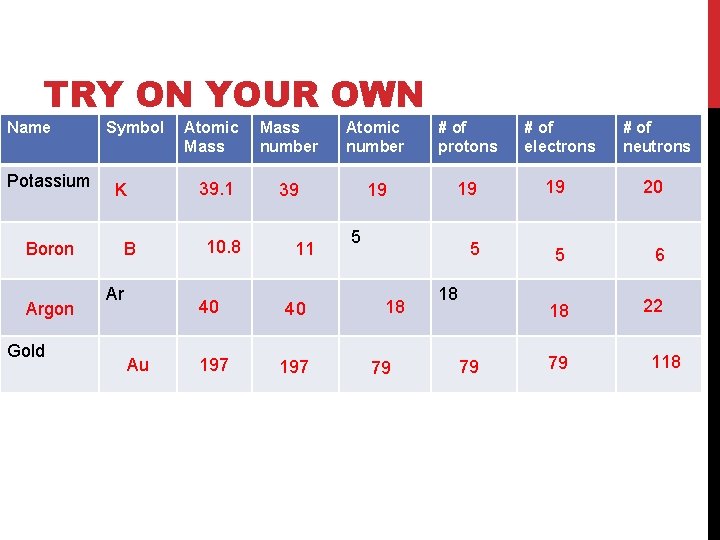
TRY ON YOUR OWN Name Potassium Boron Argon Gold Symbol Atomic Mass 39. 1 K B Ar Au 10. 8 Mass number Atomic number # of protons # of electrons # of neutrons 39 19 19 19 20 5 5 6 18 22 11 40 40 197 5 18 79 79 118
- Slides: 5