How to Prevent Li Ion Battery Failures Vidyu


How to Prevent Li. Ion Battery Failures Vidyu Challa, Ph. D Technical director, Df. R Solutions
OUTLINE Samsung 2016 Root Cause Analysis Battery failure mechanisms and modes – pathway to thermal runaway Battery protection mechanisms o o Mitigation methods o o o o 3 Internal and External Design Manufacturing Battery Management System Application Assembly, and Storage User Summary
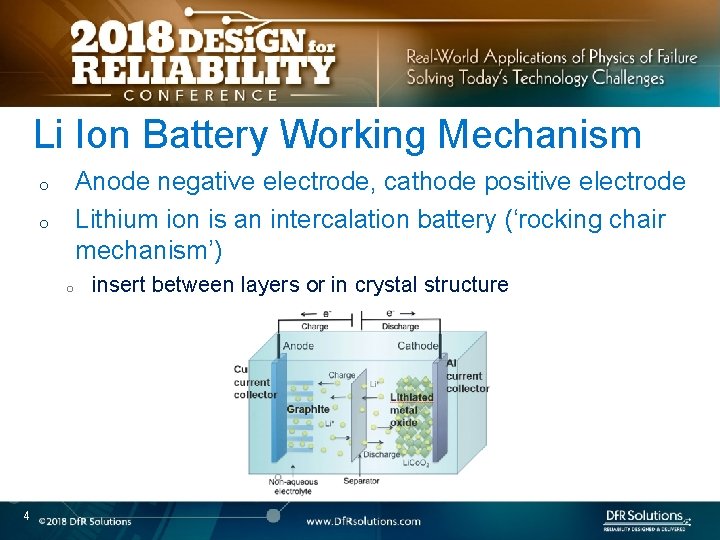
Li Ion Battery Working Mechanism Anode negative electrode, cathode positive electrode Lithium ion is an intercalation battery (‘rocking chair mechanism’) o o o 4 insert between layers or in crystal structure
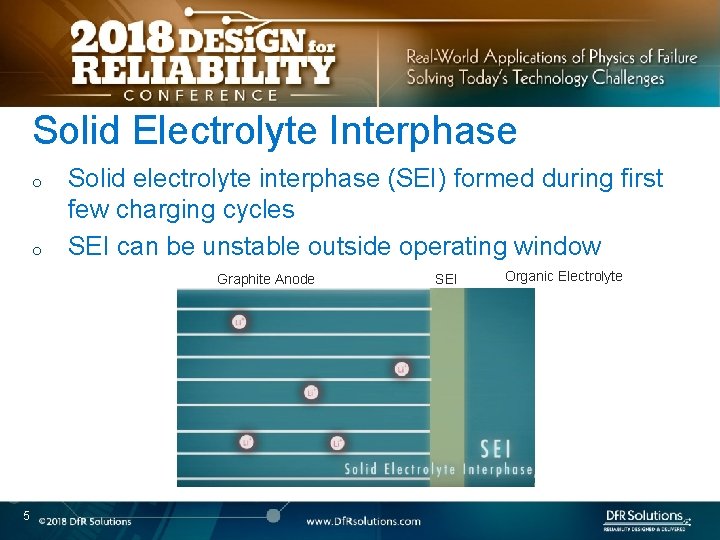
Solid Electrolyte Interphase o o Solid electrolyte interphase (SEI) formed during first few charging cycles SEI can be unstable outside operating window Graphite Anode 5 SEI Organic Electrolyte

Samsung 2016 Battery Failures Source : Samsung Galaxy Note 7 failure investigation press conference, Jan 2017. All Information in public domain 6 6

Samsung Galaxy 2016 Failures o o o 7 Galaxy Note 7 fires were reported within a few weeks of the product launch. Samsung recalled affected phones, and pointed to a manufacturing error from its battery supplier Samsung SDI. The batteries were swapped with ones from its Chinese supplier ATL. Replacement phones that were supposed to be “fixed” also started to catch on fire. Samsung scrapped the entire product line. 2. 5 million phone recalls prior. Cost Samsung $5 Billion

Flattened Jellyroll Pouch Cell Design 8
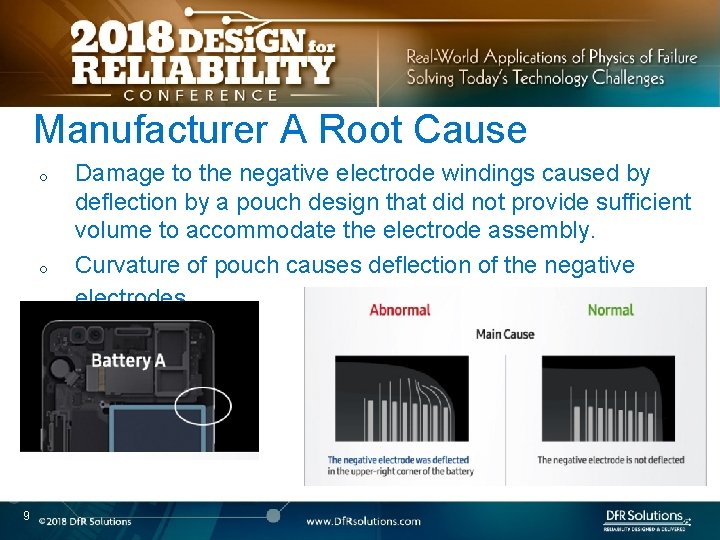
Manufacturer A Root Cause o o 9 Damage to the negative electrode windings caused by deflection by a pouch design that did not provide sufficient volume to accommodate the electrode assembly. Curvature of pouch causes deflection of the negative electrodes.
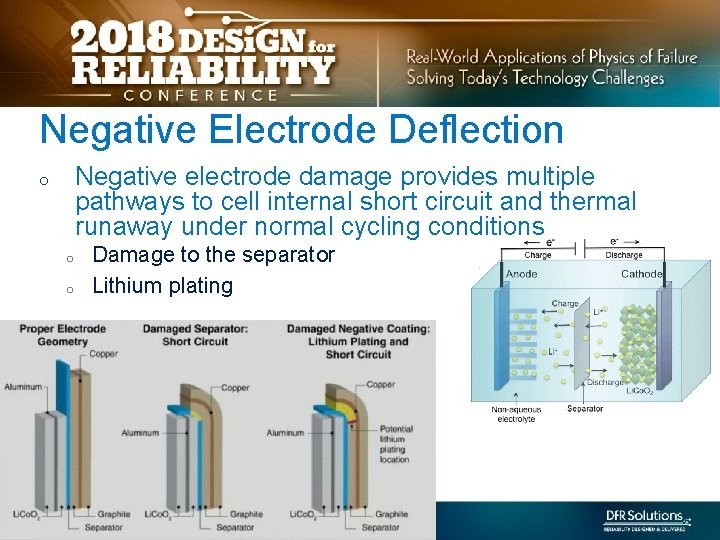
Negative Electrode Deflection Negative electrode damage provides multiple pathways to cell internal short circuit and thermal runaway under normal cycling conditions o o o Damage to the separator Lithium plating Source : Information in public domain 10

Overcrowded Bus Analogy 11
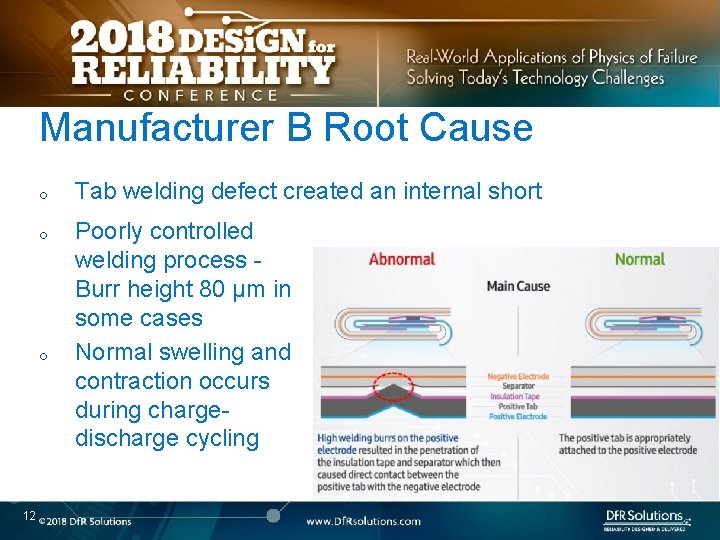
Manufacturer B Root Cause o o o 12 Tab welding defect created an internal short Poorly controlled welding process - Burr height 80 µm in some cases Normal swelling and contraction occurs during chargedischarge cycling
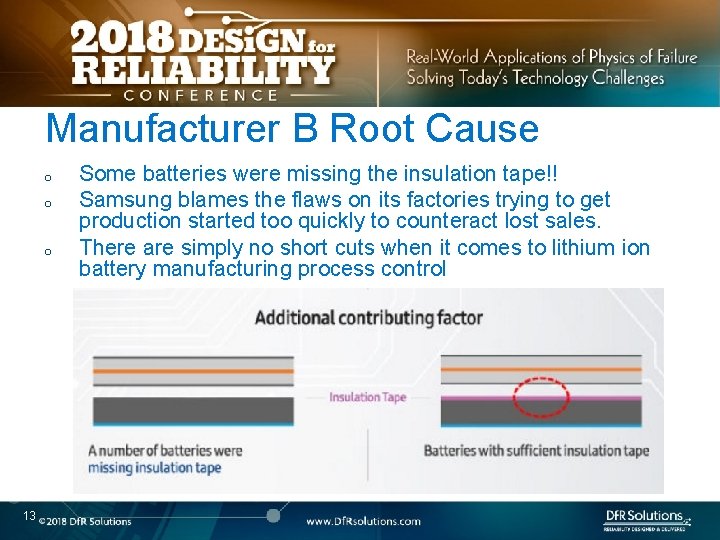
Manufacturer B Root Cause o o o 13 Some batteries were missing the insulation tape!! Samsung blames the flaws on its factories trying to get production started too quickly to counteract lost sales. There are simply no short cuts when it comes to lithium ion battery manufacturing process control
LITHIUM ION BATTERY FAILURE MODES AND MECHANISMS 14 14
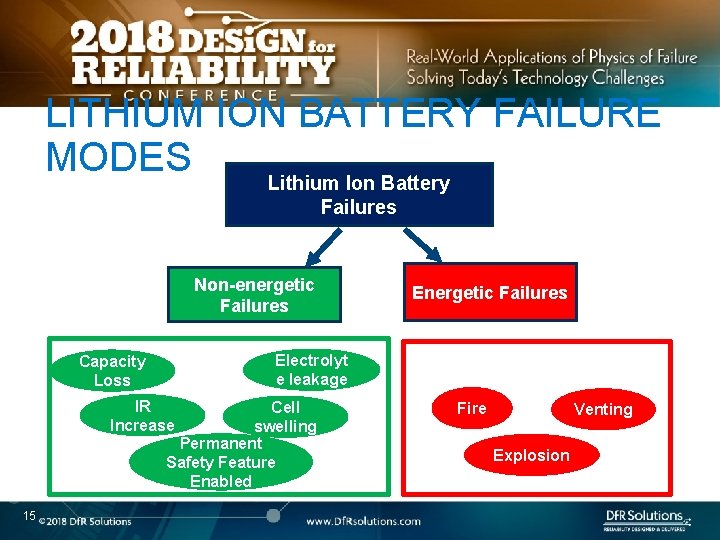
LITHIUM ION BATTERY FAILURE MODES Lithium Ion Battery Failures Non-energetic Failures Electrolyt e leakage Capacity Loss IR Increase Cell swelling Permanent Safety Feature Enabled 15 Energetic Failures Fire Venting Explosion

BENIGN, ENERGETIC AND NONENERGETIC o All non-energetic failures are not benign o What dictates whether a failure is energetic or not? o o 16 Same initiating fault can have different outcomes Depends on whether the initiating fault can create a self -sustaining exothermic reaction
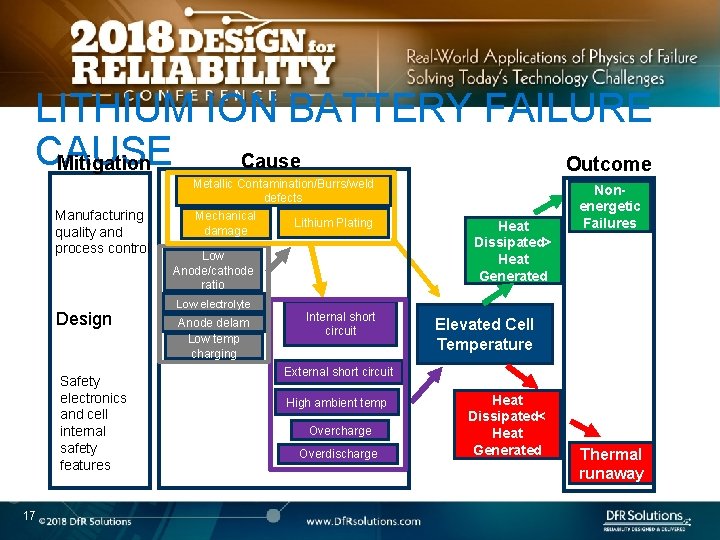
LITHIUM ION BATTERY FAILURE CAUSE Cause Mitigation Outcome Metallic Contamination/Burrs/weld defects Manufacturing quality and process control Design Safety electronics and cell internal safety features 17 Mechanical damage Lithium Plating Low Anode/cathode ratio Low electrolyte Anode delam Low temp charging Internal short circuit Heat Dissipated> Heat Generated Nonenergetic Failures Elevated Cell Temperature External short circuit High ambient temp Overcharge Overdischarge Heat Dissipated< Heat Generated Thermal runaway
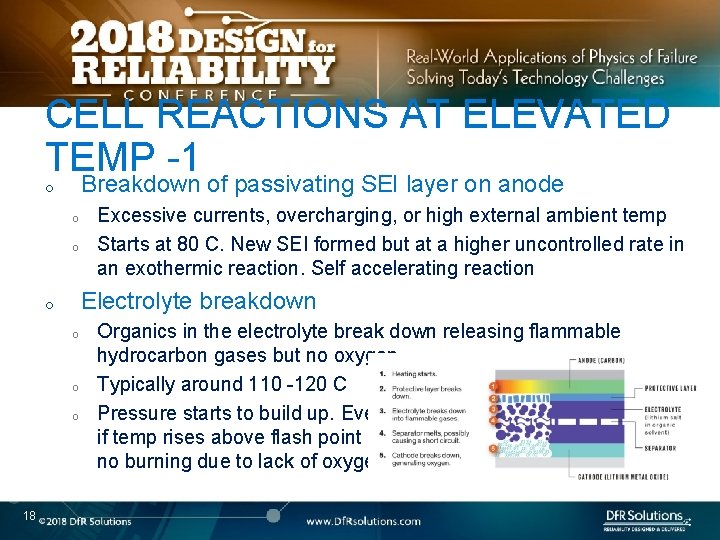
CELL REACTIONS AT ELEVATED TEMP -1 Breakdown of passivating SEI layer on anode o o o Electrolyte breakdown o o 18 Excessive currents, overcharging, or high external ambient temp Starts at 80 C. New SEI formed but at a higher uncontrolled rate in an exothermic reaction. Self accelerating reaction Organics in the electrolyte break down releasing flammable hydrocarbon gases but no oxygen. Typically around 110 -120 C Pressure starts to build up. Even if temp rises above flash point no burning due to lack of oxygen
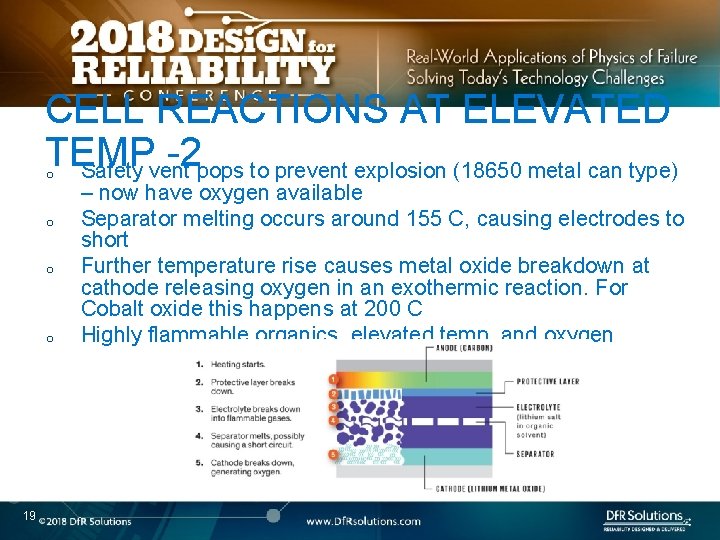
CELL REACTIONS AT ELEVATED TEMP -2 Safety vent pops to prevent explosion (18650 metal can type) o o 19 – now have oxygen available Separator melting occurs around 155 C, causing electrodes to short Further temperature rise causes metal oxide breakdown at cathode releasing oxygen in an exothermic reaction. For Cobalt oxide this happens at 200 C Highly flammable organics, elevated temp, and oxygen
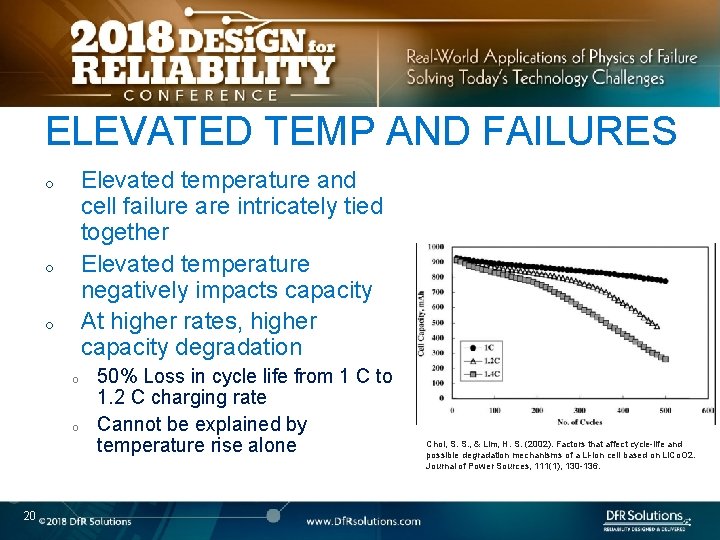
ELEVATED TEMP AND FAILURES Elevated temperature and cell failure are intricately tied together Elevated temperature negatively impacts capacity At higher rates, higher capacity degradation o o o 20 50% Loss in cycle life from 1 C to 1. 2 C charging rate Cannot be explained by temperature rise alone Choi, S. S. , & Lim, H. S. (2002). Factors that affect cycle-life and possible degradation mechanisms of a Li-ion cell based on Li. Co. O 2. Journal of Power Sources, 111(1), 130 -136.
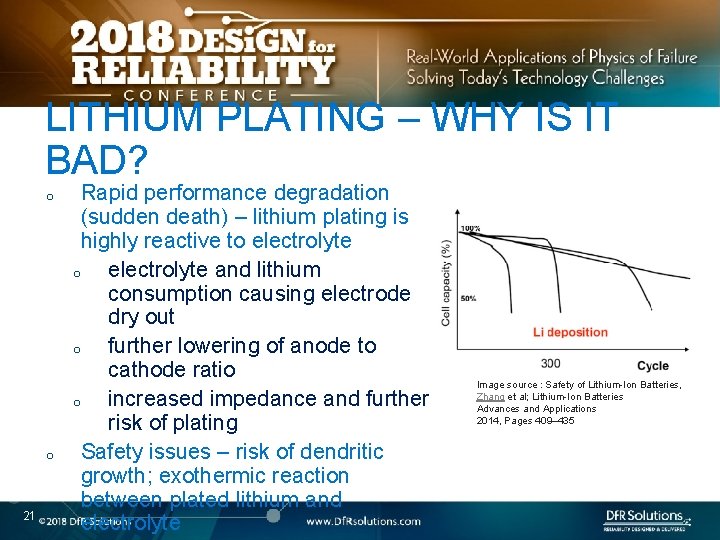
LITHIUM PLATING – WHY IS IT BAD? o o 21 Rapid performance degradation (sudden death) – lithium plating is highly reactive to electrolyte and lithium consumption causing electrode dry out o further lowering of anode to cathode ratio o increased impedance and further risk of plating Safety issues – risk of dendritic growth; exothermic reaction between plated lithium and electrolyte Image source : Safety of Lithium-Ion Batteries, Zhang et al; Lithium-Ion Batteries Advances and Applications 2014, Pages 409– 435
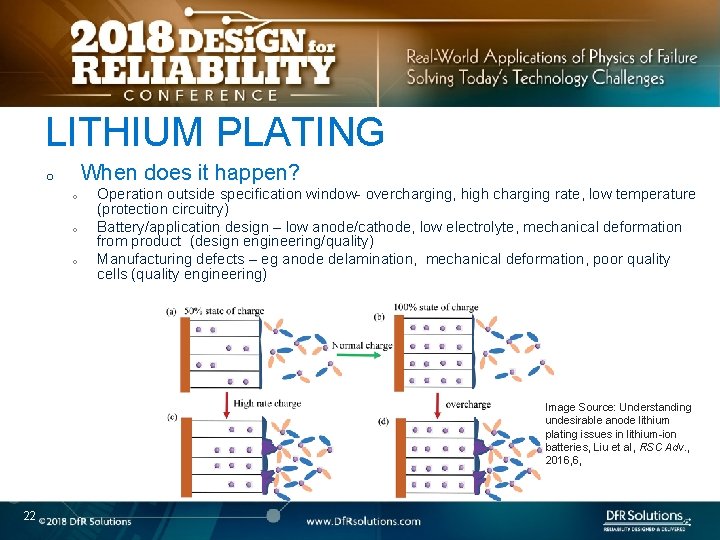
LITHIUM PLATING When does it happen? o o Operation outside specification window- overcharging, high charging rate, low temperature (protection circuitry) Battery/application design – low anode/cathode, low electrolyte, mechanical deformation from product (design engineering/quality) Manufacturing defects – eg anode delamination, mechanical deformation, poor quality cells (quality engineering) Image Source: Understanding undesirable anode lithium plating issues in lithium-ion batteries, Liu et al, RSC Adv. , 2016, 6, 22
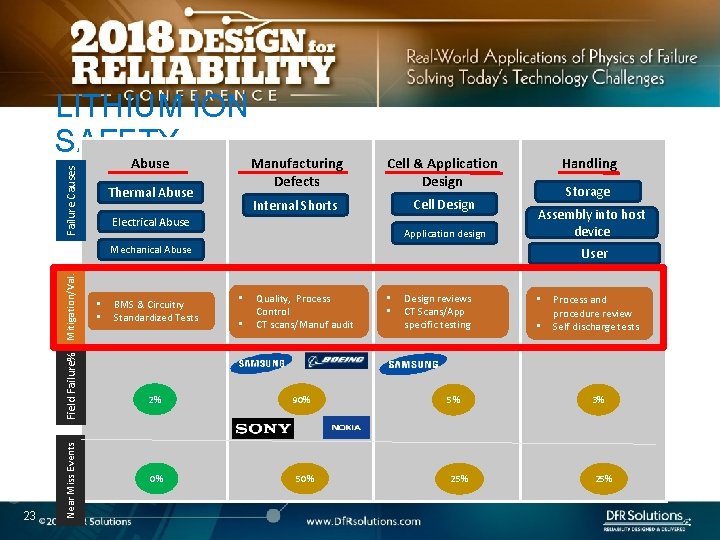
LITHIUM ION SAFETY Failure Causes Abuse Thermal Abuse Manufacturing Defects Cell & Application Design Internal Shorts Cell Design Electrical Abuse Application design Handling Storage Assembly into host device 23 Near Miss Events Field Failure% Mitigation/Val. Mechanical Abuse • • BMS & Circuitry Standardized Tests User • • Quality, Process Control CT scans/Manuf audit • • Design reviews CT Scans/App specific testing • • Process and procedure review Self discharge tests 2% 90% 5% 3% 0% 50% 25%

Internal Shorts – ‘Soft underbelly’ Internal shorts are typically the result of manufacturing defects and are not effectively mitigated by safety systems There is no good internal short circuit test that effectively screens out these defects The best defense is to have sound manufacturing process controls 24 24
Improper BMS Design o Poorly designed BMS and use of improper chargers are responsible for some of the spectacular field failures o o Battery protection system includes the charger circuitry, protection circuit module located at the battery, and is optimized for a specific cell chemistry and application o 25 Over-voltage failures are more spectacular and immediate than grown in manufacturing defects which can take time to manifest themselves Good BMS design should include use of a custom connector so that everyday USB chargers can’t be plugged in and allowed to abuse the cell

LITHIUM ION CELL MANUFACTURING PROCESS 26 26

MANUFACTURING CONTROLS 27 o Incoming raw materials o Electrode fabrication and cell assembly o Environmental control
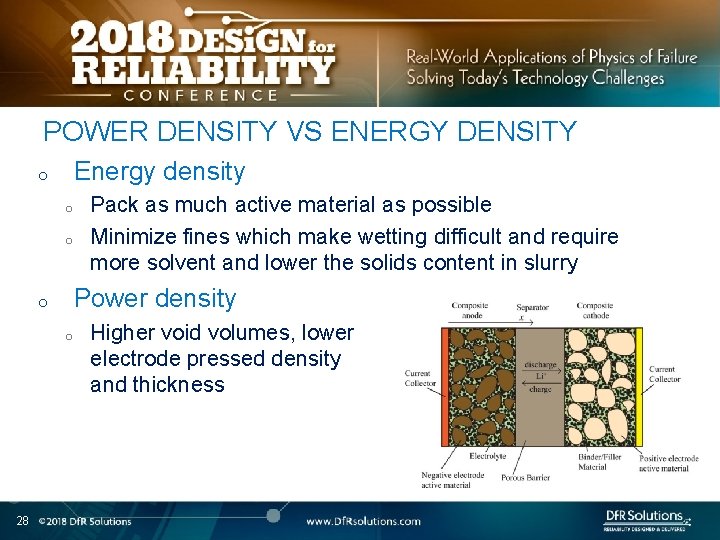
POWER DENSITY VS ENERGY DENSITY Energy density o o o Power density o o 28 Pack as much active material as possible Minimize fines which make wetting difficult and require more solvent and lower the solids content in slurry Higher void volumes, lower electrode pressed density and thickness
MANUFACTURING INCOMING RAW MATERIALS o o o 29 Incoming powders – Tap density, particle size distribution (PSD), surface area, purity Binders and binder solvents Separator Electrolyte Current collectors Dry room storage (dew point of at least -40 C). Dew point is used to express water vapor concentration at this level because the corresponding relative humidity value is less than 1%.
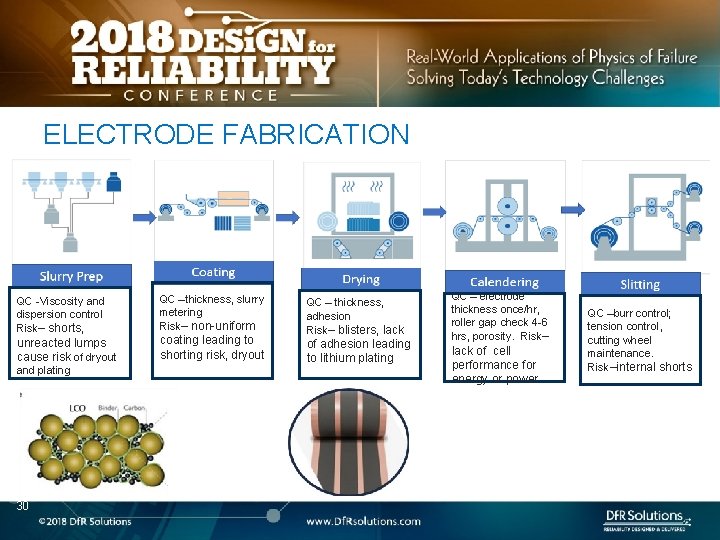
ELECTRODE FABRICATION QC -Viscosity and dispersion control Risk– shorts, unreacted lumps cause risk of dryout and plating 30 QC –thickness, slurry metering Risk– non-uniform coating leading to shorting risk, dryout QC – thickness, adhesion Risk– blisters, lack of adhesion leading to lithium plating QC – electrode thickness once/hr, roller gap check 4 -6 hrs, porosity. Risk– lack of cell performance for energy or power QC –burr control; tension control, cutting wheel maintenance. Risk–internal shorts
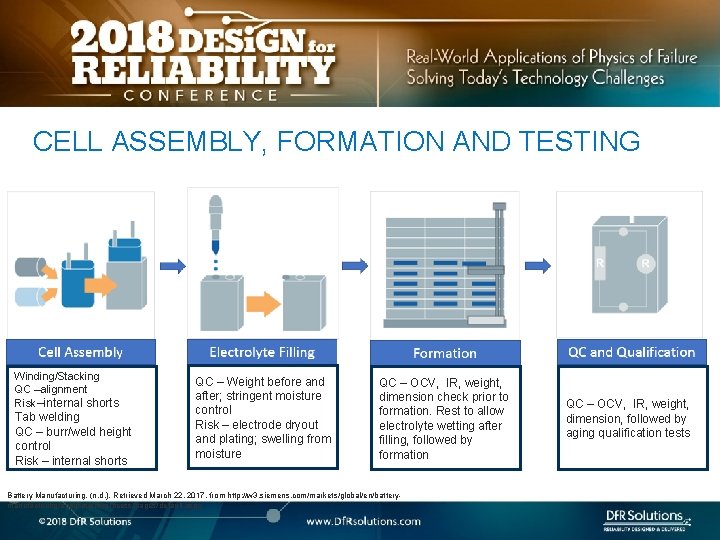
CELL ASSEMBLY, FORMATION AND TESTING Winding/Stacking QC –alignment Risk–internal shorts Tab welding QC – burr/weld height control Risk – internal shorts QC – Weight before and after; stringent moisture control Risk – electrode dryout and plating; swelling from moisture QC – OCV, IR, weight, dimension check prior to formation. Rest to allow electrolyte wetting after filling, followed by formation Battery Manufacturing. (n. d. ). Retrieved March 22, 2017, from http: //w 3. siemens. com/markets/global/en/batterymanufacturing/applications/process/pages/default. aspx QC – OCV, IR, weight, dimension, followed by aging qualification tests
DRYING TEMPERATURES The process involves a multi-zone oven with low temperatures for the first zone, higher temperatures for the middle zone, followed by a lower temperature for the final zone. o o o 32 Specific temperatures will depend on binder chemistry and on slurry properties. For PVDF binder on the cathode, the recommended drying oven temperature profile for a 5 -zone oven is as follows: zone 1 – 75 -90 °C; zones 2 -4 – 125 -130 ° C; zone 5 – approximately 75 ° C. A 4 -zone oven should run at slower speed to allow more solvent evaporation.

GENERAL MANUFACTURING GUIDELINES Incoming materials control o o 33 Internal specifications that identify, minimize and control all known and likely impurities in incoming materials Internal specifications that control material properties or specs Ability to track changes in vendor materials

GENERAL MANUFACTURING GUIDELINES Manufacturing process control o o o 34 Safety critical equipment must be identified - process of verifying equipment operation periodically. Preventive maintenance plan implementation Procedures to avoid metal contamination throughout the manufacturing process Processes to collect loose material, such as coating dust A method of detecting mechanical damage to electrodes in the manufacturing process, such as an automated vision system Statistical process control (SPC) to monitor maximum particle size, slurry viscosities, coating weight, calendered thickness, weight of electrolyte dispensed, cell weight, open circuit voltage (OCV) and capacity
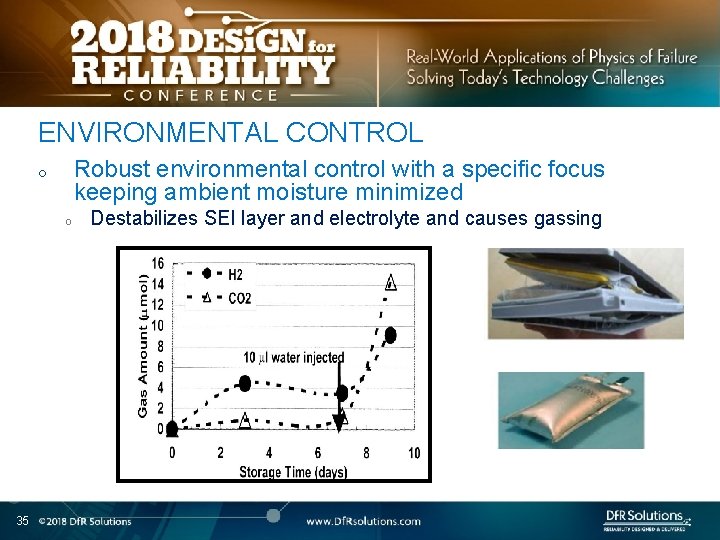
ENVIRONMENTAL CONTROL Robust environmental control with a specific focus keeping ambient moisture minimized o o 35 Destabilizes SEI layer and electrolyte and causes gassing

LITHIUM ION CELL DESIGN GUIDELINES 36 36
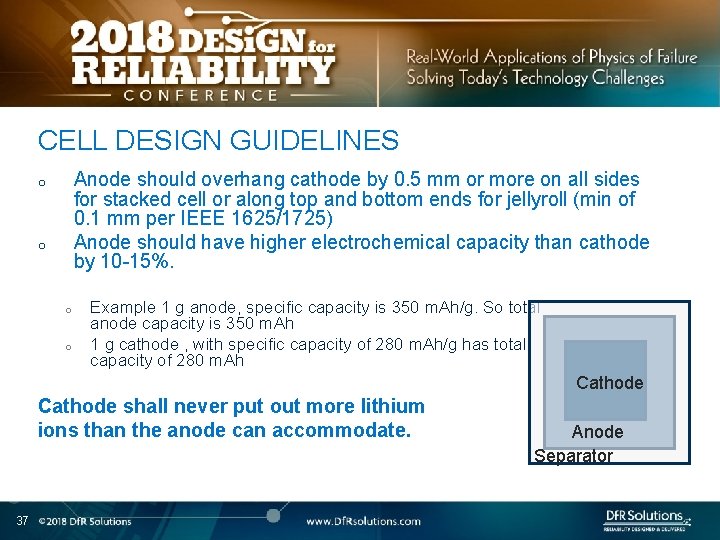
CELL DESIGN GUIDELINES Anode should overhang cathode by 0. 5 mm or more on all sides for stacked cell or along top and bottom ends for jellyroll (min of 0. 1 mm per IEEE 1625/1725) Anode should have higher electrochemical capacity than cathode by 10 -15%. o o Example 1 g anode, specific capacity is 350 m. Ah/g. So total anode capacity is 350 m. Ah 1 g cathode , with specific capacity of 280 m. Ah/g has total capacity of 280 m. Ah Cathode shall never put out more lithium ions than the anode can accommodate. 37 Anode Separator
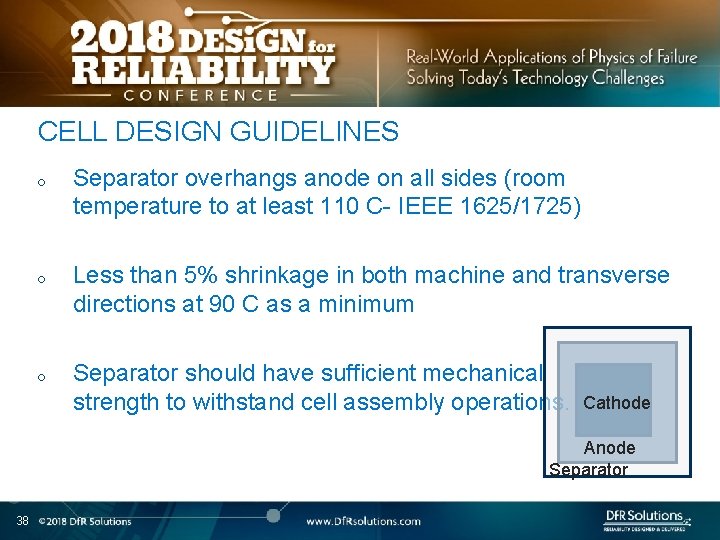
CELL DESIGN GUIDELINES o o o Separator overhangs anode on all sides (room temperature to at least 110 C- IEEE 1625/1725) Less than 5% shrinkage in both machine and transverse directions at 90 C as a minimum Separator should have sufficient mechanical strength to withstand cell assembly operations. Cathode Anode Separator 38

CELL DESIGN GUIDELINES o Product design – make sure there is enough room for battery to expand as it goes through charge –discharge cycles. Recommend 10 % margin. o Pay particular attention to corners Poor manufacturing can reduce design margin o o o 39 Cutting operation variability Pick and place variability
ARTICLE QUALITY ASESSMENT : CELL CT SCANS AND TEARDOWN 40 40
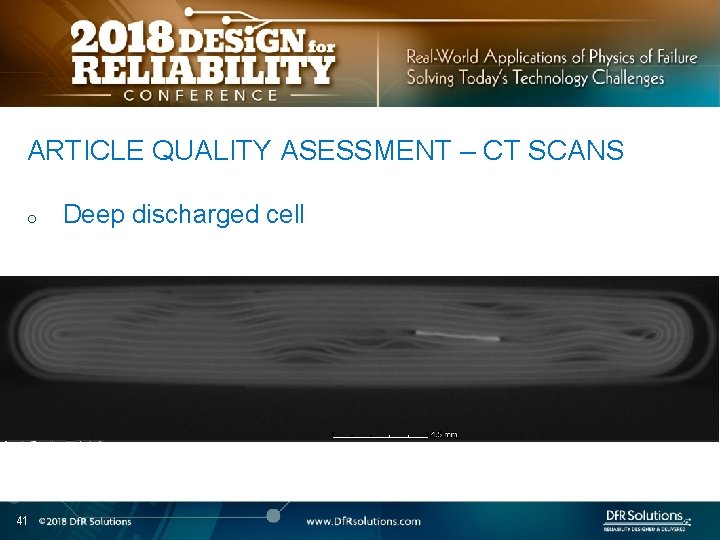
ARTICLE QUALITY ASESSMENT – CT SCANS o 41 Deep discharged cell

ARTICLE QUALITY ASESSMENT- CT SCANS o o 42 Exercise - Describe what you see Electrode alignment is good on right image Variability in jellyroll winding on left and middle images Insufficient anode overlap in center of middle image
PHYSICAL ARTICLE QUALITY ASESSMENT – CT SCANS Conduct CT scans and cell teardowns on a handful of units What to look for in CT scans o o o o 43 Uniform cell winding or stacking No particulates No significant electrode delamination Anodes should overhang cathode by 0. 5 mm ideally but a minimum of 0. 1 mm on each side No excessive deformation at pouch corners o Cutting operation/pick and place variability
ARTICLE QUALITY ASESSMENT – TEARDOWNS o 44 Look at a few electrode pieces using optical microscopy and SEM/EDS

LITHIUM ION INTERNAL AND EXTERNAL PROTECTION SYSTEMS 45 45
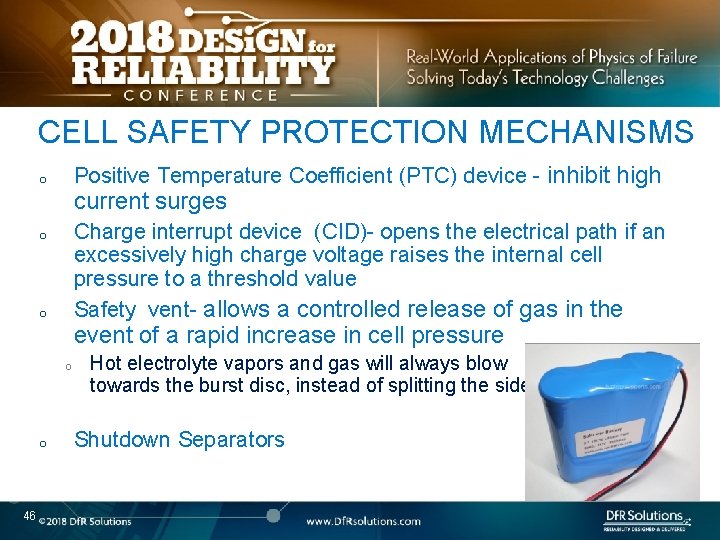
CELL SAFETY PROTECTION MECHANISMS Positive Temperature Coefficient (PTC) device - inhibit high o current surges Charge interrupt device (CID)- opens the electrical path if an excessively high charge voltage raises the internal cell pressure to a threshold value Safety vent- allows a controlled release of gas in the o o event of a rapid increase in cell pressure o o 46 Hot electrolyte vapors and gas will always blow towards the burst disc, instead of splitting the sides Shutdown Separators
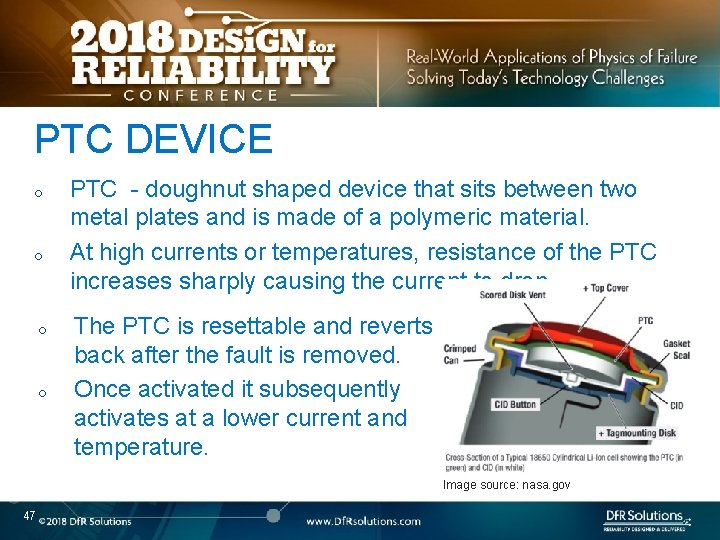
PTC DEVICE o o PTC - doughnut shaped device that sits between two metal plates and is made of a polymeric material. At high currents or temperatures, resistance of the PTC increases sharply causing the current to drop. The PTC is resettable and reverts back after the fault is removed. Once activated it subsequently activates at a lower current and temperature. Image source: nasa. gov 47
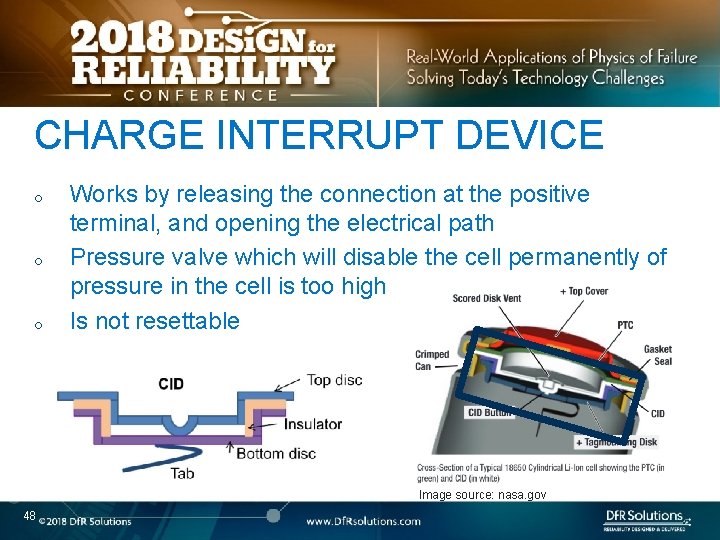
CHARGE INTERRUPT DEVICE o o o Works by releasing the connection at the positive terminal, and opening the electrical path Pressure valve which will disable the cell permanently of pressure in the cell is too high Is not resettable Image source: nasa. gov 48
POUCH CELL PROTECTION o o Pouch cells do not have an internal CID, PTC or safety vent Can have PTC or fuse elements in series with cell Image source: nasa. gov 49
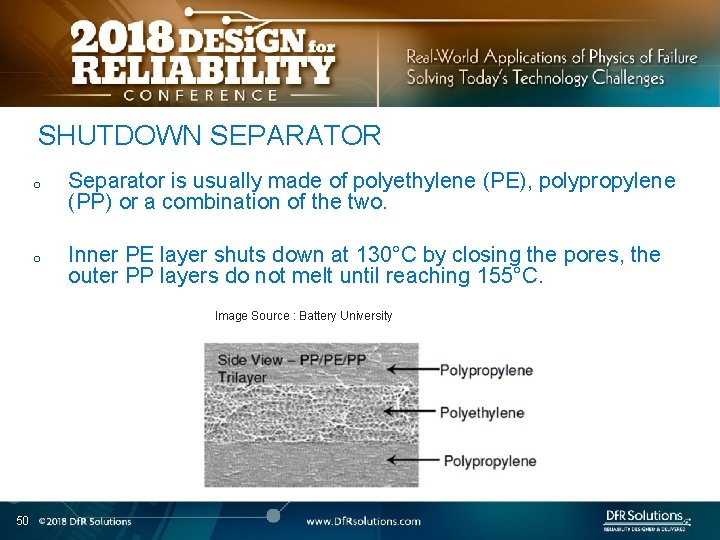
SHUTDOWN SEPARATOR o Separator is usually made of polyethylene (PE), polypropylene (PP) or a combination of the two. o Inner PE layer shuts down at 130°C by closing the pores, the outer PP layers do not melt until reaching 155°C. Image Source : Battery University 50
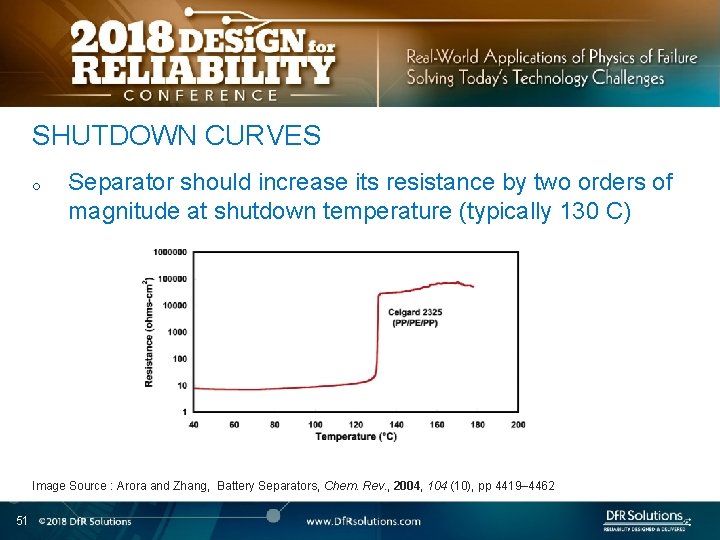
SHUTDOWN CURVES o Separator should increase its resistance by two orders of magnitude at shutdown temperature (typically 130 C) Image Source : Arora and Zhang, Battery Separators, Chem. Rev. , 2004, 104 (10), pp 4419– 4462 51
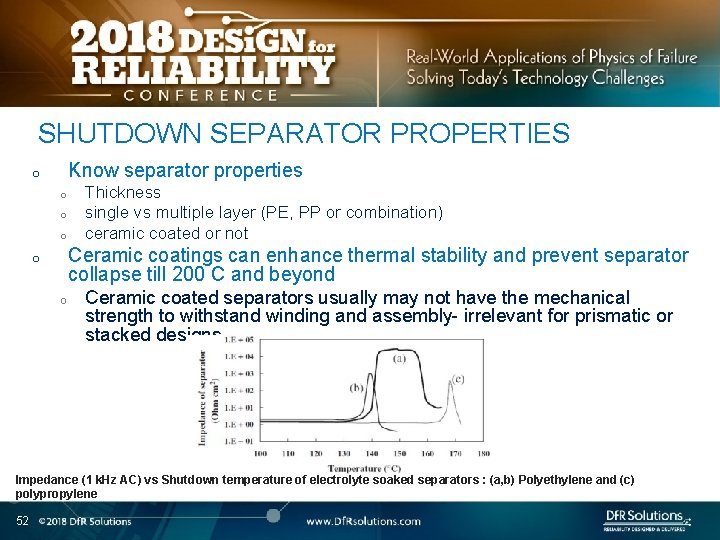
SHUTDOWN SEPARATOR PROPERTIES Know separator properties o o Thickness single vs multiple layer (PE, PP or combination) ceramic coated or not Ceramic coatings can enhance thermal stability and prevent separator collapse till 200 C and beyond o o Ceramic coated separators usually may not have the mechanical strength to withstand winding and assembly- irrelevant for prismatic or stacked designs Impedance (1 k. Hz AC) vs Shutdown temperature of electrolyte soaked separators : (a, b) Polyethylene and (c) polypropylene 52

BATTERY MANAGEMENT SYSTEM (BMS) AND PROTECTION CIRCUITRY 53 53
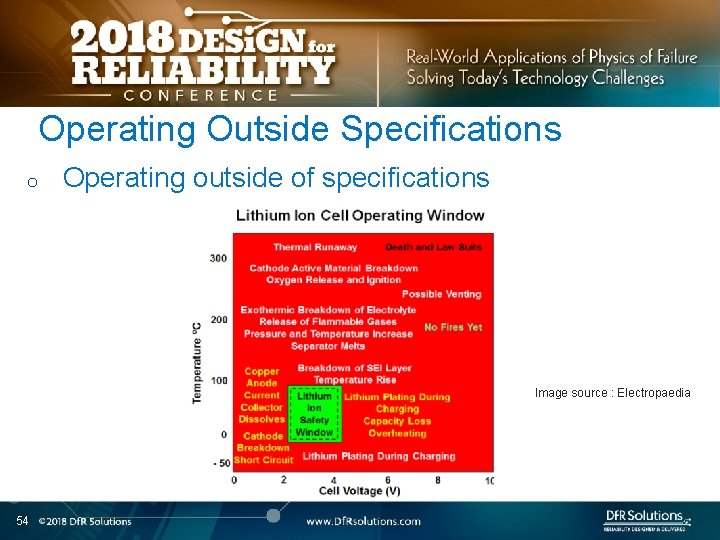
Operating Outside Specifications o Operating outside of specifications Image source : Electropaedia 54

Severity of Thermal Runaway o Depends on a number of factors o o o Most severe thermal runaway occurs in an overcharged state o o 55 State of charge (SOC) Ambient temperature Cell chemistry Cell design Case temperatures can reach 600 C. High temperatures are driven by exothermic reactions of the electrodes and the electrolyte
Thermal Runaway Initiation – Some Numbers Self heating temperature o o o Overcharge o o 56 Fully charged 18650 cells brought to self heating temperature (70 to 90 C) in adiabatic environment go into thermal runaway in 2 days. Fully charged 18650 in adiabatic environment brought to 150 C (with separator melting) will run away in minutes UL standard requires fully charged cells to withstand 4 hour storage at 75 C, and 10 min storage at 130 C. IEEE standard requires 1 hour exposure at 130 C Charging a 4. 2 V system to 5 V will almost certainly cause immediate thermal runaway

EV Bus Failure o o EV electric bus caught fire in Shenzhen, China during charging BMS Failure Without a BMS, there is no safe lithium ion battery o 57
ELECTRONIC PROTECTION SYSTEMS Consist of charger and a battery protection printed circuit board (PCB) o o 58 Also called a battery protection circuit module (PCM) Important functions of protection systems include monitoring, controlling and terminating charge and discharge as needed. Failure of protection circuitry to either sense or respond to an out of range condition, causes battery failure

BMS FUNCTIONS Maintain cells within operating window and prevent cells from going into o o Over voltage Over current Over temperature Over discharge Balance individual cells to enhance overall capacity o Disconnect batteries in a safe way in emergency situations o BMS Webinar : https: //www. dfrsolutions. com/battery-management-systems-and-safety-and-reliability-webinar Predict remaining capacity or state of charge o 59
OVER CHARGE - VOLTAGE Most obvious failure mode is exceeding specified voltage o o Charging a 4. 2 V system to 5 V will almost certainly cause immediate thermal runaway o Charging at excess currents o Overcharge effects o o 60 Anode – lithium plating rather than intercalation Cathode –excess de-intercalation causes crystal structure to collapse and release heat Heat and gas release (both Joule heating and parasitic reactions) Electrolyte and electrode decomposition
OVER VOLTAGE Over voltage may be applied to the battery from a defective charger or due to improper monitoring on a single cell o o 61 Slight overcharge (from minor deviations in voltage monitoring) causes capacity loss rather than direct thermal runaway. Overvoltage protection can be implemented by opening a charge MOSFET or a fuse Overcharge protection is so critical that multiple independent circuits are typically used to prevent single points of failure
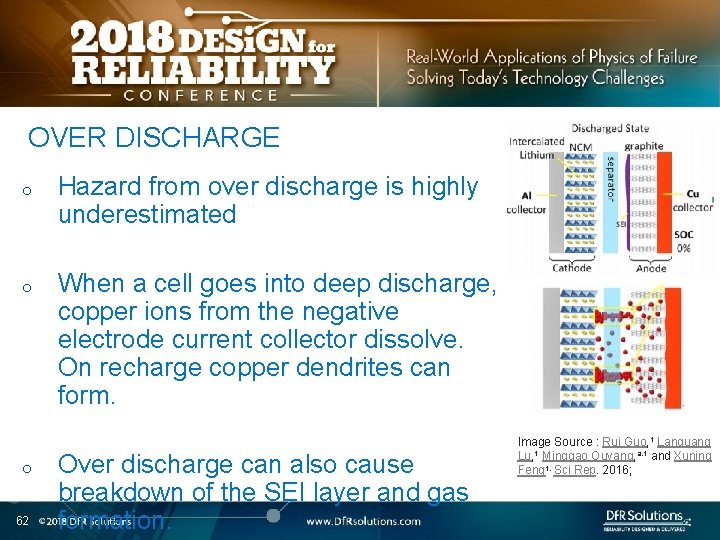
OVER DISCHARGE o o o 62 Hazard from over discharge is highly underestimated When a cell goes into deep discharge, copper ions from the negative electrode current collector dissolve. On recharge copper dendrites can form. Over discharge can also cause breakdown of the SEI layer and gas formation. Image Source : Rui Guo, 1 Languang Lu, 1 Minggao Ouyang, a, 1 and Xuning Feng 1, Sci Rep. 2016;

How Does a Battery Go into Deep Discharge? 63 63
OVER DISCHARGE SCENARIOS o o 64 Uncontrolled storage without appropriate recharge procedures Mechanically damaged cells (from electronics impinging on cell)

Discharge During Storage Three kinds of discharge occur during storage o o o 65 PCM sleep current and leakage currents Battery self discharge o Manufacturing quality o Temp o State of charge Device sleep currents At 3 V (or other threshold cutoff), load is disconnected although above ‘consumption currents’ are active. At deep discharge cutoff value, of 2 V (or other manufacturer specified value) PCM and device sleep currents are turned off, but battery self discharge continues.
Battery Self Discharge Lithium ion self discharge around 1 -2% per month + a few % cent for PCM o High SOC and temperatures degrade storage life (and cycle life) o o o 66 Rule of thumb – 10 C increase in temperature doubles self discharge rate Store at conditions close to 25 C and 40 % SOC

PCM (BMS) Self Discharge o o o 67 To keep the contamination as low as possible to reduce leakage current, de-ionized water wash should be used in the board cleaning process Verify the board cleanliness to make sure the levels of contamination is low Investigate if humidity is an issue and that conformal needs to be used

Deep Discharge Checklist Have a pre-check charging function on batteries where deep discharge cutoff voltage was reached. Charger circuit checks if a deeply discharged battery is reaches a threshold value in give time. Otherwise considered a ‘damaged’ battery o o o 68 Mitigating hazard from copper shunts Proper battery storage and recharge procedures Minimize PCM leakage currents Ensure cell quality Prevent cell mechanical damage

OVER CURRENT PROTECTIONS Over current can cause o o o 69 Degradation in cycle life Thermal runaway Threshold limits for current are functions of both current and time. Protection circuit system can use different combinations of time and current to produce a fault. Example - high current/short time or lower current/long time. Some faults are recoverable such as when a MOSFET is turned on, and others are non-recoverable such as when a fuse is ignited. Causes of over-current could be a defective charger, or due to an internal short.
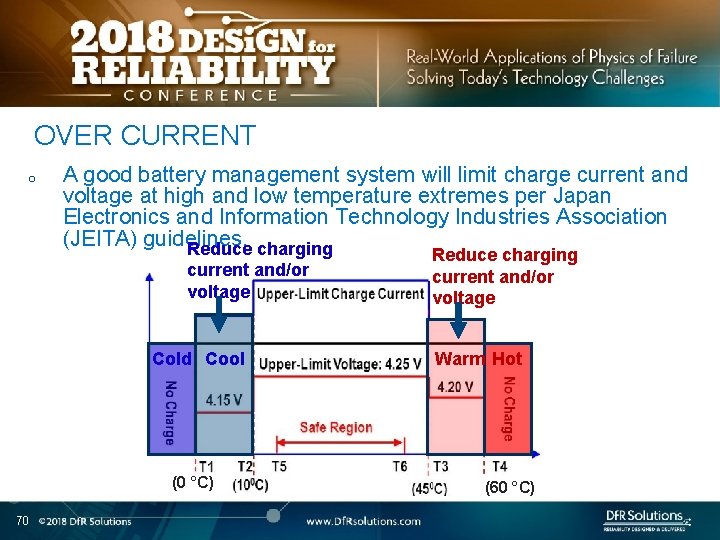
OVER CURRENT o A good battery management system will limit charge current and voltage at high and low temperature extremes per Japan Electronics and Information Technology Industries Association (JEITA) guidelines. Reduce charging current and/or voltage Cold Cool (0 °C) 70 Reduce charging current and/or voltage Warm Hot (60 °C)
SHORT CIRCUIT PROTECTION o Current limits are incorporated into the protection circuits located in pack or device o o 71 Circuits monitor current in and out of battery and open up MOSFET to interrupt current Backup protection usually includes a fuse or PTC placed in series with battery pack
BATTERY LIFE, DEGRADATION AND STORAGE 72 72
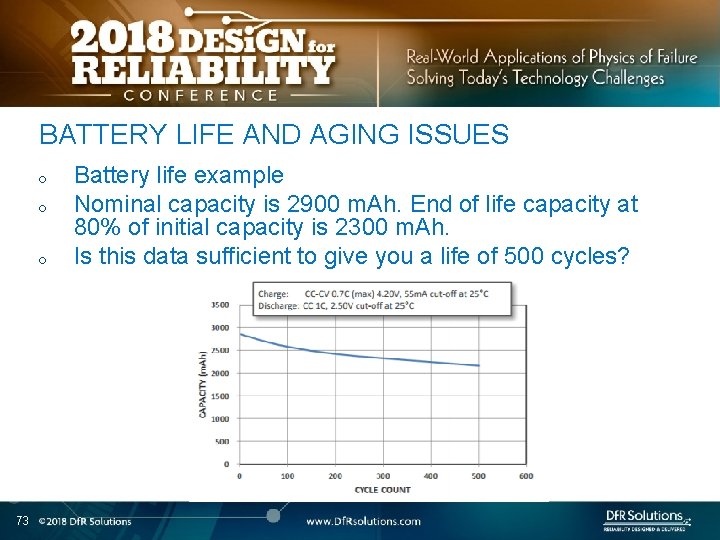
BATTERY LIFE AND AGING ISSUES o o o 73 Battery life example Nominal capacity is 2900 m. Ah. End of life capacity at 80% of initial capacity is 2300 m. Ah. Is this data sufficient to give you a life of 500 cycles?
BATTERY LIFE DEGRADATION Calendar aging vs cycle life aging o o o 74 Insertion or ejection of the Lithium ions into and out of the intercalation spaces during cycling causes the electrode materials to swell or contract. Repetitive cycling can weaken the electrode structure reducing its adhesion to the current collector. This can lead to reduction in charge capacity.
BATTERY LIFE DEGRADATION: CALENDAR AGING o Storage degradation : high temperature aging causes growth of the passivating layer o o 75 It consumes lithium and electrolyte and leads to capacity loss and impedance increase (both capacity and power fade). Pores can be blocked as a result. Low rate batteries may not see a big impact (voltage drop = IR)
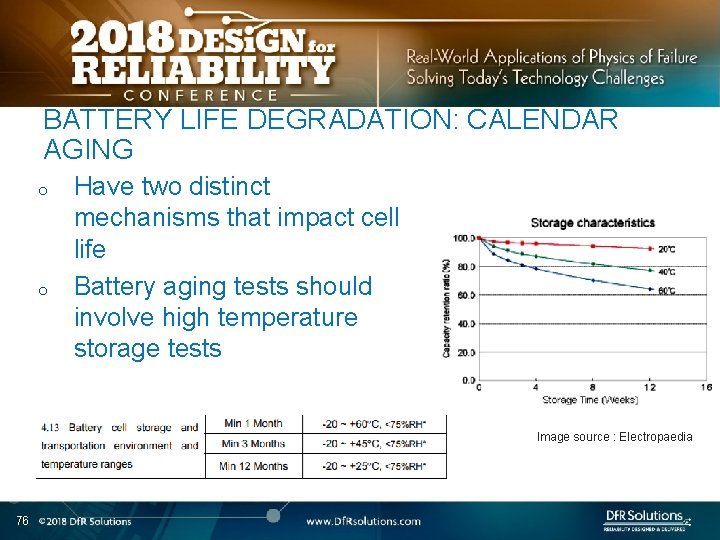
BATTERY LIFE DEGRADATION: CALENDAR AGING o o Have two distinct mechanisms that impact cell life Battery aging tests should involve high temperature storage tests Image source : Electropaedia 76
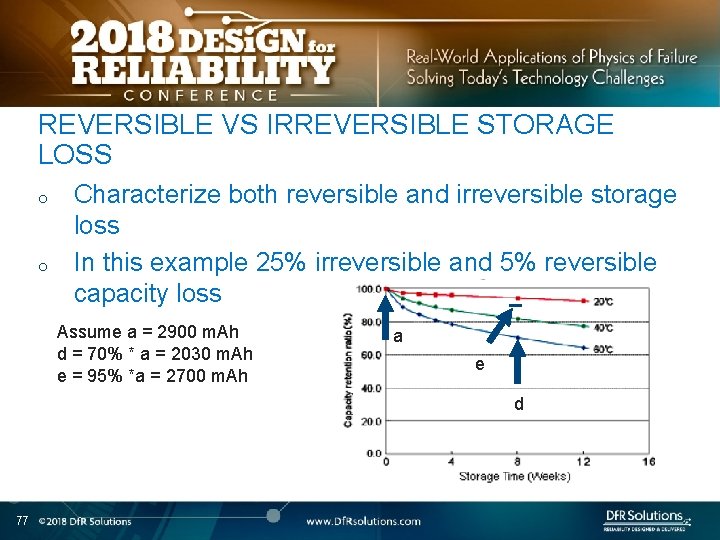
REVERSIBLE VS IRREVERSIBLE STORAGE LOSS o o Characterize both reversible and irreversible storage loss In this example 25% irreversible and 5% reversible capacity loss Assume a = 2900 m. Ah d = 70% * a = 2030 m. Ah e = 95% *a = 2700 m. Ah a e d 77

LITHIUM ION CHEMISTRIES 78 78
Battery Chemistry Term Lithium-Ion encompasses many different chemistries Anode – graphite (Lithium Titanate LTO for very fast charge cells) Cathode - Cathodes are lithiated metal oxides or lithiated metal phosphates o o o o 79 Lithium Cobalt Oxide (LCO) Lithium Iron Phosphate(LFP) Nickel Manganese Cobalt (NMC) Lithium Manganese Oxide(LMO) Cathode chemistry is where you get enhancements in safety, energy and power density
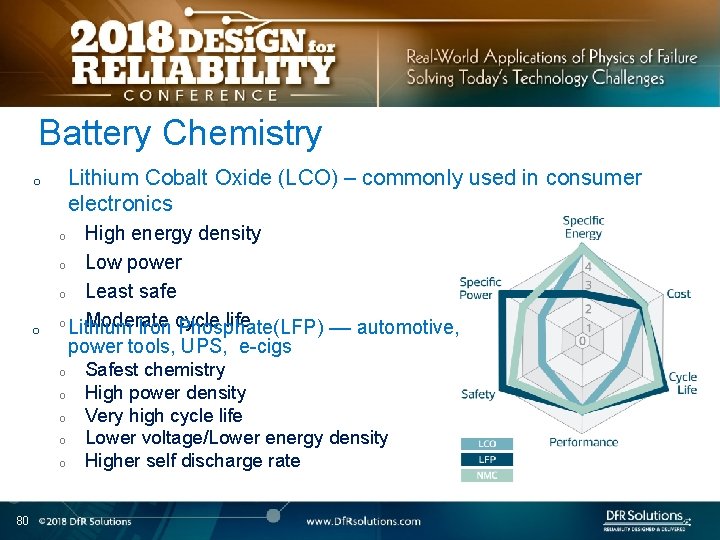
Battery Chemistry Lithium Cobalt Oxide (LCO) – commonly used in consumer electronics o High energy density o Low power o Least safe o Moderate cycle life Lithium Iron Phosphate(LFP) –– automotive, power tools, UPS, e-cigs o o o o 80 Safest chemistry High power density Very high cycle life Lower voltage/Lower energy density Higher self discharge rate
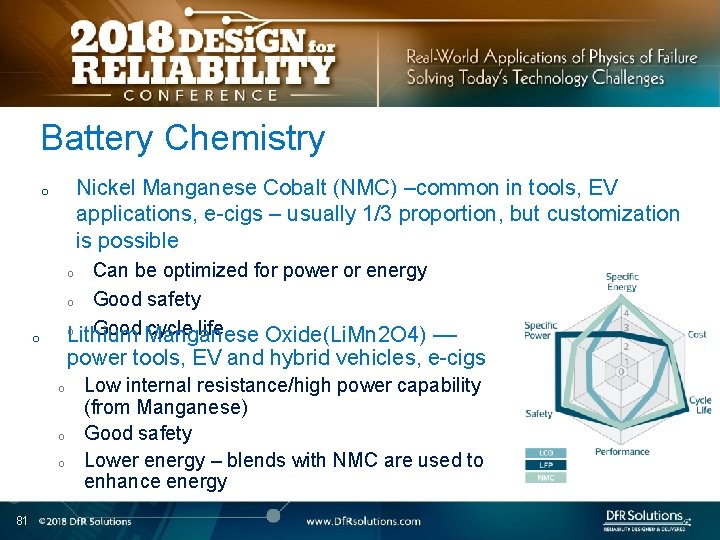
Battery Chemistry Nickel Manganese Cobalt (NMC) –common in tools, EV applications, e-cigs – usually 1/3 proportion, but customization is possible o Can be optimized for power or energy o Good safety o Good cycle life Lithium Manganese Oxide(Li. Mn 2 O 4) –– o o power tools, EV and hybrid vehicles, e-cigs o o o 81 Low internal resistance/high power capability (from Manganese) Good safety Lower energy – blends with NMC are used to enhance energy
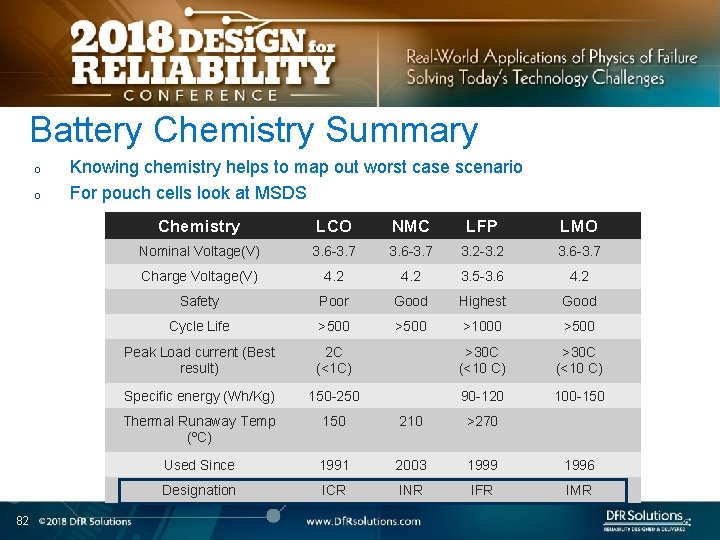
Battery Chemistry Summary o o 82 Knowing chemistry helps to map out worst case scenario For pouch cells look at MSDS Chemistry LCO NMC LFP LMO Nominal Voltage(V) 3. 6 -3. 7 3. 2 -3. 2 3. 6 -3. 7 Charge Voltage(V) 4. 2 3. 5 -3. 6 4. 2 Safety Poor Good Highest Good Cycle Life >500 >1000 >500 Peak Load current (Best result) 2 C (<1 C) >30 C (<10 C) Specific energy (Wh/Kg) 150 -250 90 -120 100 -150 Thermal Runaway Temp (ºC) 150 210 >270 Used Since 1991 2003 1999 1996 Designation ICR INR IFR IMR
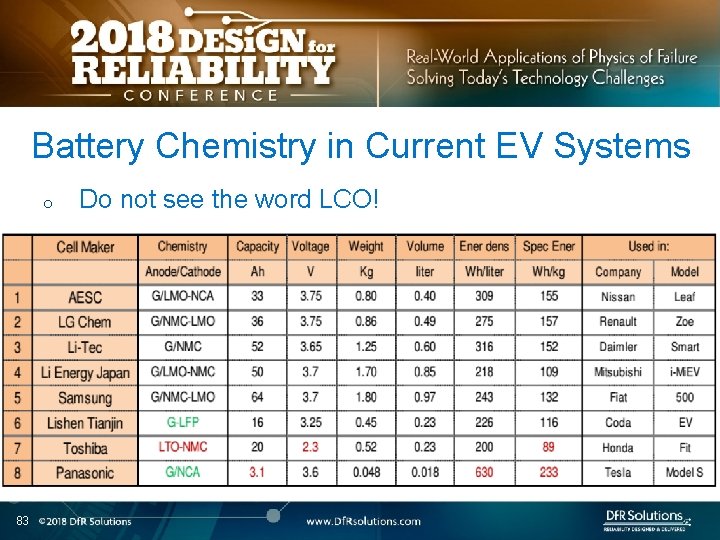
Battery Chemistry in Current EV Systems o 83 Do not see the word LCO!

ESTIMATING STORED ENERGY 84 84

BATTERY RISK ASESSMENT Risk assessment o o o Risk mitigation o o 85 How much energy and wattage does the battery have? How fast is the energy released? Is the product worn on or close to the body? What is the chemistry? New product category : Are there specific user behaviors/product design interactions that increase the risk and impact of thermal away? Trust but verify Do not trust till you verify Map out the worst case

STORED ENERGY Stored energy is a combination of electrical and chemical energy o o o 86 Electrical Energy Chemical energy may be approximated by using heats of combustion of various flammable components in cell.
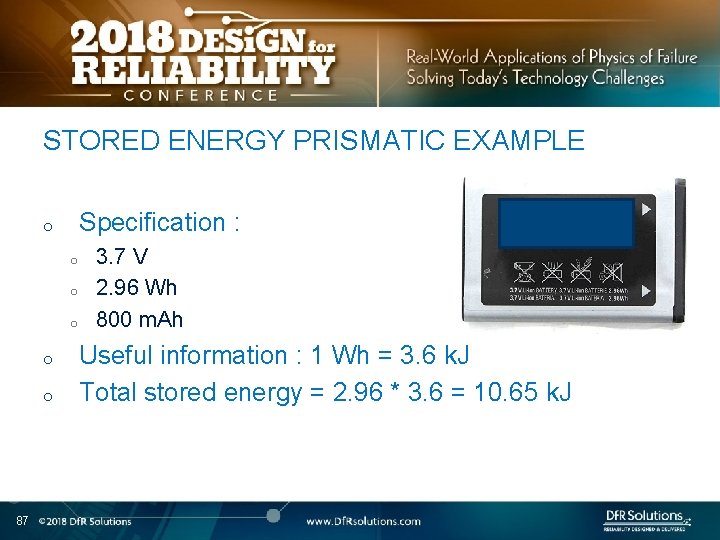
STORED ENERGY PRISMATIC EXAMPLE Specification : o o o 87 3. 7 V 2. 96 Wh 800 m. Ah Useful information : 1 Wh = 3. 6 k. J Total stored energy = 2. 96 * 3. 6 = 10. 65 k. J
STORED CHEMICAL ENERGY: SMALL PRISMATIC Assume 1 to 5 g of electrolyte for small prismatic or pouch cell. Assume 0. 5 to 1 g of separator. Electrolyte solvents are generally organic carbonates, such as diethyl carbonate (DEC) , ethylene carbonate, dimethyl carbonate, and ethylmethyl carbonate. o o o Separator is made of polyethylene, polypropylene or combination of the two. o o o 88 Using the heat of combustion of DEC at 20. 92 k. J/g, 21 to 105 k. J of energy from electrolyte combustion By using polypropylene heat of combustion as an approximation at 42. 66 k. J/g, 21 to 42 k. J energy for the separator Total chemical energy approximation is 42 to 147 k. J
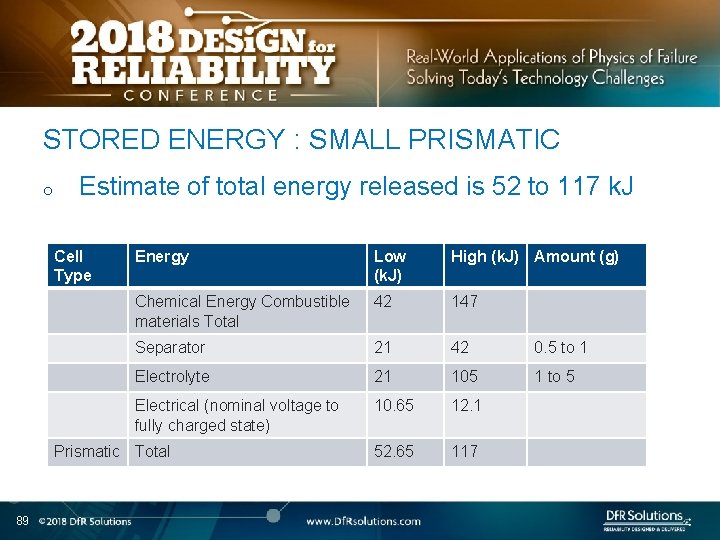
STORED ENERGY : SMALL PRISMATIC o Estimate of total energy released is 52 to 117 k. J Cell Type Energy Low (k. J) High (k. J) Amount (g) Chemical Energy Combustible materials Total 42 147 Separator 21 42 0. 5 to 1 Electrolyte 21 105 1 to 5 Electrical (nominal voltage to fully charged state) 10. 65 12. 1 52. 65 117 Prismatic Total 89
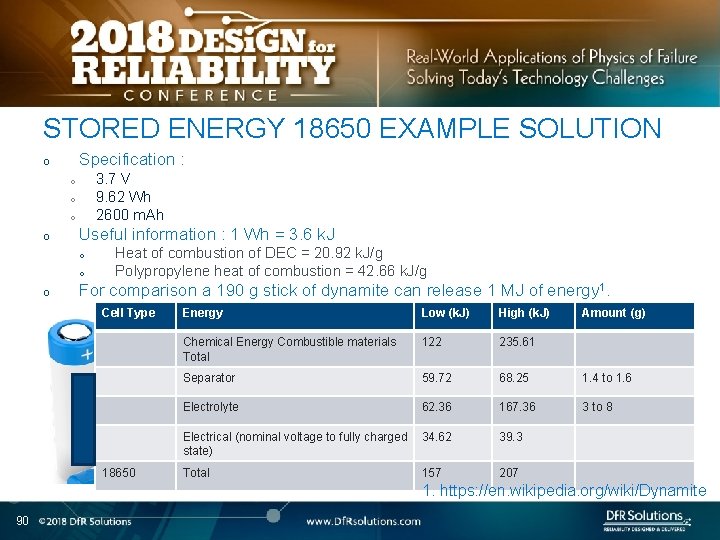
STORED ENERGY 18650 EXAMPLE SOLUTION Specification : o 3. 7 V 9. 62 Wh 2600 m. Ah o o Useful information : 1 Wh = 3. 6 k. J o o o Heat of combustion of DEC = 20. 92 k. J/g Polypropylene heat of combustion = 42. 66 k. J/g For comparison a 190 g stick of dynamite can release 1 MJ of energy 1. Cell Type 18650 Energy Low (k. J) High (k. J) Chemical Energy Combustible materials Total 122 235. 61 Separator 59. 72 68. 25 1. 4 to 1. 6 Electrolyte 62. 36 167. 36 3 to 8 Electrical (nominal voltage to fully charged 34. 62 state) 39. 3 Total 207 157 Amount (g) 1. https: //en. wikipedia. org/wiki/Dynamite 90

Is the 18650 Hazard Relevant to You? 91 91

Small Batteries Smaller batteries - 20 m. Ah to 500 m. Ah Energy released is 10 - to 100 times lower than the 18650 o o 92 Smaller capacity batteries release smaller amounts of energy 150 -200 m. Ah batteries generally used for headphone applications such as in illustration
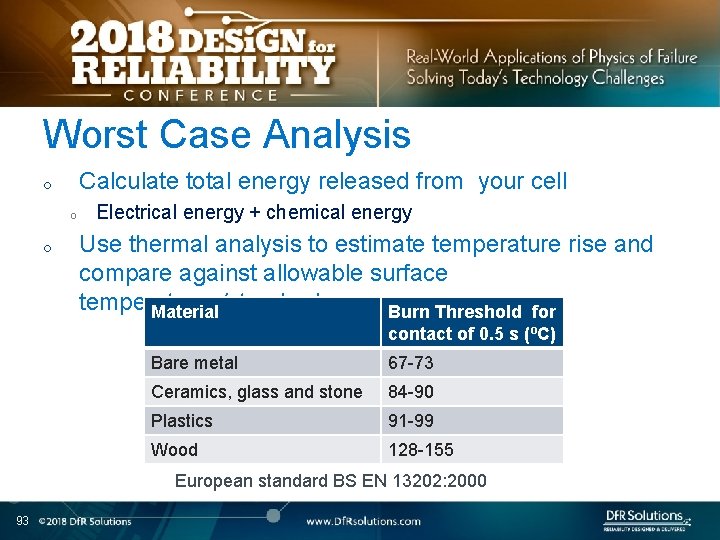
Worst Case Analysis Calculate total energy released from your cell o o o Electrical energy + chemical energy Use thermal analysis to estimate temperature rise and compare against allowable surface temperatures/standards Material Burn Threshold for contact of 0. 5 s (ºC) Bare metal 67 -73 Ceramics, glass and stone 84 -90 Plastics 91 -99 Wood 128 -155 European standard BS EN 13202: 2000 93
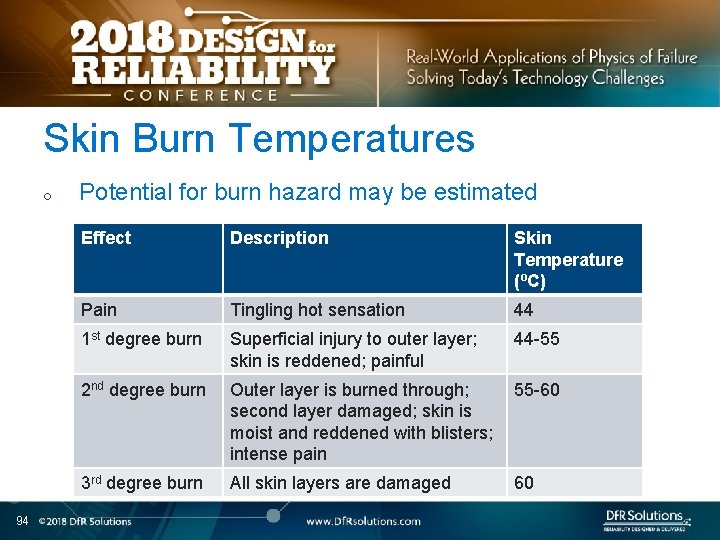
Skin Burn Temperatures o 94 Potential for burn hazard may be estimated Effect Description Skin Temperature (ºC) Pain Tingling hot sensation 44 1 st degree burn Superficial injury to outer layer; skin is reddened; painful 44 -55 2 nd degree burn Outer layer is burned through; 55 -60 second layer damaged; skin is moist and reddened with blisters; intense pain 3 rd degree burn All skin layers are damaged 60

VENT GAS RISK 95 95
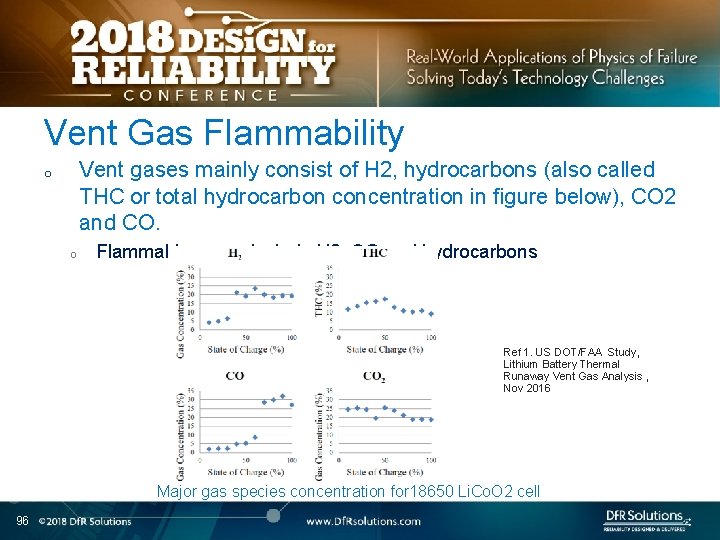
Vent Gas Flammability Vent gases mainly consist of H 2, hydrocarbons (also called THC or total hydrocarbon concentration in figure below), CO 2 and CO. o o Flammable gases include H 2, CO and hydrocarbons Ref 1. US DOT/FAA Study, Lithium Battery Thermal Runaway Vent Gas Analysis , Nov 2016 Major gas species concentration for 18650 Li. Co. O 2 cell 96

Ignition Risk o When a lithium ion battery vents, the gas mixture will mix with surrounding air and may or may not ignite o The following conditions have to be met for an ignition event o o 97 Air fuel mixture is within the Lower Flammability Limit (LFL) and (Upper Flammability Limit) UFL limits Ignition source is present The most dangerous materials are those with the lowest flash point and widest flammable ranges. A hot cell case or hot metal sparks ejected from the cell could create an ignition event, if the mixture is within flammability limits.

CHEMICAL SPILL RISK 98 98

Chemical Spill Risk In the absence of a fire, potential hazard from a damaged lithium ion battery includes the following: o o o Release of a electrolyte containing a corrosive salt. Electrolyte is extremely corrosive and may cause permanent blindness. If ingested through the mouth, liver and kidney damage is possible. Reaction of the electrolyte with water/humidity may generate hydrofluoric acid which are highly toxic and corrosive to the eyes, nose, throat and skin. Release of volatile organics, toxic gases such as CO, HF. Ref. Various MSDS sheets o 99

Chemical Risk – Lithium Ion and Primary Lithium Lithiated carbon in a charged anode, the Solid Electrolyte Interphase (SEI) layer or any free lithium (dendrites/plating) will burst into flames when exposed to moist air o Reaction of lithium with water produces H 2 in an exothermic reaction. o o 100 Significant heat is released in this reaction, and this can ignite the H 2.
Lithium Ion Life Cycle – Cradle to Grave 101
LITHIUM ION BATTERY LIFE CYCLE Cell Manufacturing o o o o 102 brought to a low to moderate state of charge for shipping/storage Transportation Warehouse storage Pack or device assembly OEM device shipment Device Usage Recycling

Transportation Procedures Almost all failures are related to improper packaging (potential for mechanical damage and external shorts) and shipment procedures. o International Air Transportation Association (IATA) shipment procedures for dangerous goods. Cell manufacturers must show proof of UN 38. 3 certification o o 1) Lithium ion batteries (not contained in equipment) shall not be shipped with SOC greater than 30 %. 2) Shall pass UN 38. 3 battery of tests 3) Shall not be shipped on passenger aircraft See http: //www. iata. org/whatwedo/cargo/dgr/Documents/lithium-battery-shipping-guidelines. pdf 103
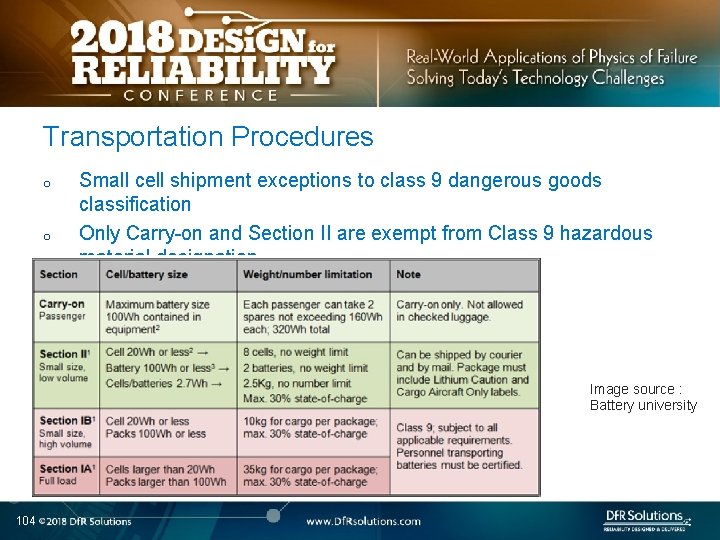
Transportation Procedures o o Small cell shipment exceptions to class 9 dangerous goods classification Only Carry-on and Section II are exempt from Class 9 hazardous material designation. Image source : Battery university 104
Storage and Warehouse Handling o o o 105 Have procedures in place for safe handling of lithium ion batteries Protection from short circuit, high temperature is critical Pouch cells must use recessed packaging trays

Lithium Ion Recycling Procedures o Do not single stream recycle lithium ion batteries o o o 106 o High risk of ignition with surrounding paper and cardboard Lithium Ion batteries can be recycled, but only at specified locations. Visit https: //www. call 2 recycle. org/locator/ (Rechargeable Battery Recycling Corporation) 86% of US and Canadian residents live within 10 miles of drop-off location https: //www. call 2 recycle. org for battery recycling

Lithium Ion Recycling Procedures Do not discard as trash When collecting batteries, make sure they are taped or insulated o o o 107 Recommend drop-off instead

COMPLIANCE TESTING 108
BATTERY COMPLIANCE TESTING o o o 109 Tests that have the same name under different standards are not always the same. There are differences in terms of the state of charge, aging of the cells and sample sizes and these can have significant differences. The vendor must state the test standard, specify a test description in addition to the name of the test.
BATTERY COMPLIANCE TESTING UN/DOT 38. 3. Covers transportation safety testing for all lithium metal and lithium ion cells and batteries. This is mandatory. o UL safety standards o o 110 UL 1642 – This standard is used for testing lithium cells. Battery level tests are covered by UL 2054 (Household and Commercial Batteries) – For lithium batteries, UL 2054 defers all component cell level testing to UL 1642. CEI/IEC 62133 – this standard is voluntary in the US, but some countries specify this standard.

BATTERY COMPLIANCE TESTING o o o 111 IEEE 1625/1725 – These standards are applicable to rechargeable batteries for Multi-cell mobile computing devices and for Cellular phones respectively (CTIA or Wireless Association). These standards take the most comprehensive approach to battery testing, and emphasize that battery pack safety is a function of a) the individual cells b) the battery pack, c) the host device, d) power supply accessories e) the user f) the environment. Both standards require design analysis tools such as FMEA or fault tree analysis. They also encompass industry best practices in manufacturing, and in the areas of cell, pack, system, and charging accessory design.

Parting Thought The more you know the better! 112

Questions? • Contact Vidyu Challa, vchalla@dfrsolutions. com, 301 -640 -5834 • www. dfrsolutions. com resources page for battery and other electronic reliability resources

APPENDIX 114

Battery Seal Quality Moisture ingress through o o Compensate through large seal width ( generally 2 mm min) o o 115 Laminate face (extremely low due to metal barrier) Interfaces (no metal barrier) Terminals (good sealing practices mandatory) Seal width checks
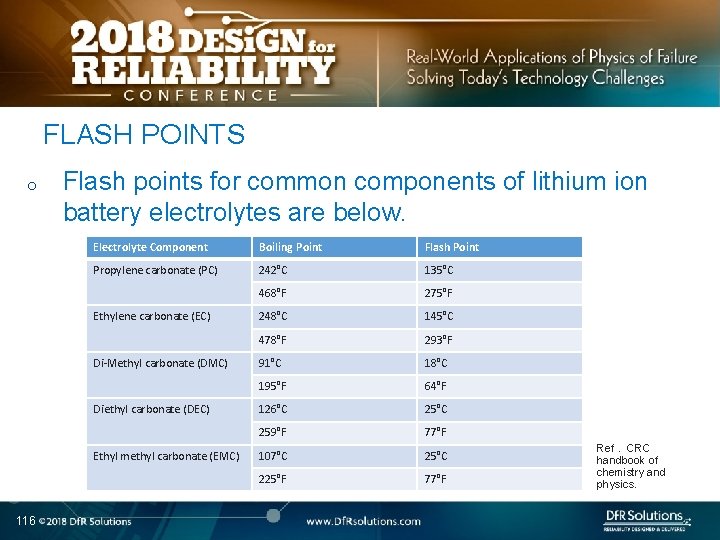
FLASH POINTS o Flash points for common components of lithium ion battery electrolytes are below. Electrolyte Component Boiling Point Flash Point Propylene carbonate (PC) 242°C 135°C 468°F 275°F 248°C 145°C 478°F 293°F 91°C 18°C 195°F 64°F 126°C 259°F 77°F 107°C 25°C 225°F 77°F Ethylene carbonate (EC) Di-Methyl carbonate (DMC) Diethyl carbonate (DEC) Ethyl methyl carbonate (EMC) 116 Ref. CRC handbook of chemistry and physics.
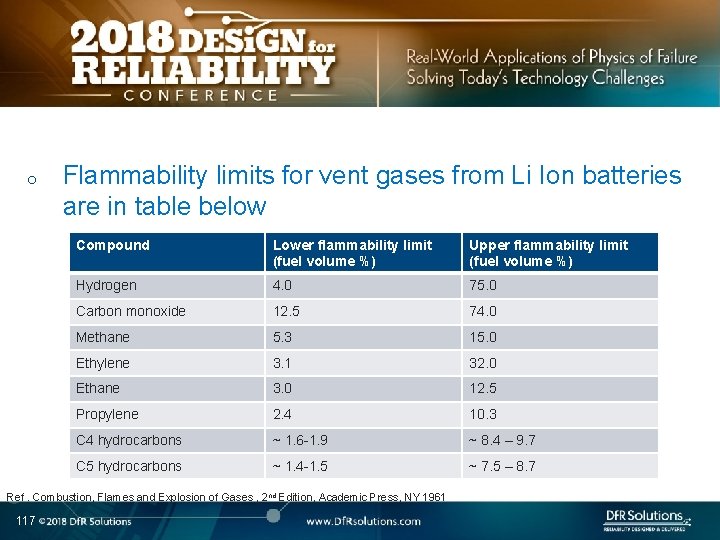
o Flammability limits for vent gases from Li Ion batteries are in table below Compound Lower flammability limit (fuel volume %) Upper flammability limit (fuel volume %) Hydrogen 4. 0 75. 0 Carbon monoxide 12. 5 74. 0 Methane 5. 3 15. 0 Ethylene 3. 1 32. 0 Ethane 3. 0 12. 5 Propylene 2. 4 10. 3 C 4 hydrocarbons ~ 1. 6 -1. 9 ~ 8. 4 – 9. 7 C 5 hydrocarbons ~ 1. 4 -1. 5 ~ 7. 5 – 8. 7 Ref. Combustion, Flames and Explosion of Gases , 2 nd Edition, Academic Press, NY 1961 117
- Slides: 117