How to plan and conduct data management Database


목차 • How to plan and conduct data management? - Database Design & Considerations - How to make a electronic Case Report Form(e. CRF) - Data Validation

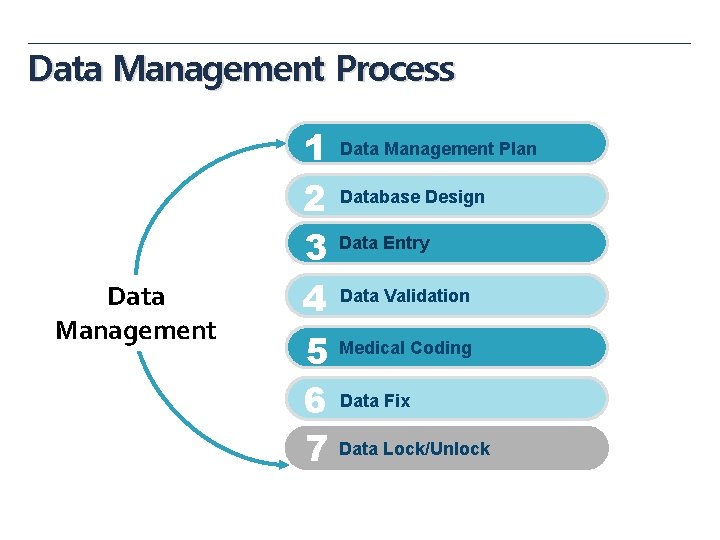
Data Management Process Data Management 1 Data Management Plan 2 Database Design 3 Data Entry 4 Data Validation 5 Medical Coding 6 Data Fix 7 Data Lock/Unlock

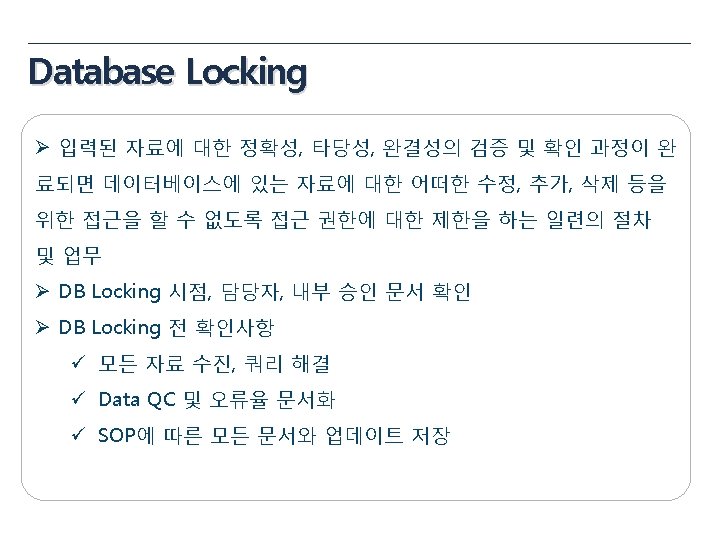
Data Management Plan(DMP) Ø Data Management Process 정의 문서 Ø 연구에 적용되는 SOPs과 Guideline 명시 Ø 과제 담당자의 규정(자격)과 역할 Ø 산출물과 문서화할 내용에 대한 명시 Ø Database Design Ø Data Entry Rule Ø Data Validation Rule Ø Medical Coding Ø SAE Reconciliation Ø Data Quality Contrl(QC) Ø Electronic Data Archiving

DMP 구성 Ø 준비단계 ü Document control ü Study personnel ü Timelines ü Communication and status reporting


Timeline Ø Time & Quality Metrics ü draft DMP, approved DMP and revised DMP ü Database test and QC report ü Data check program and Test ü Start to issue Data Clarification Form(DCF) ü Final queries issued to site ü Coding Complete ü Data Quality Control(QC) Report

DMP 구성 Ø 진행단계 ü Database ü Security & Backup ü Data Entry ü Non-CRF data upload ü Data validation ü Data Coding




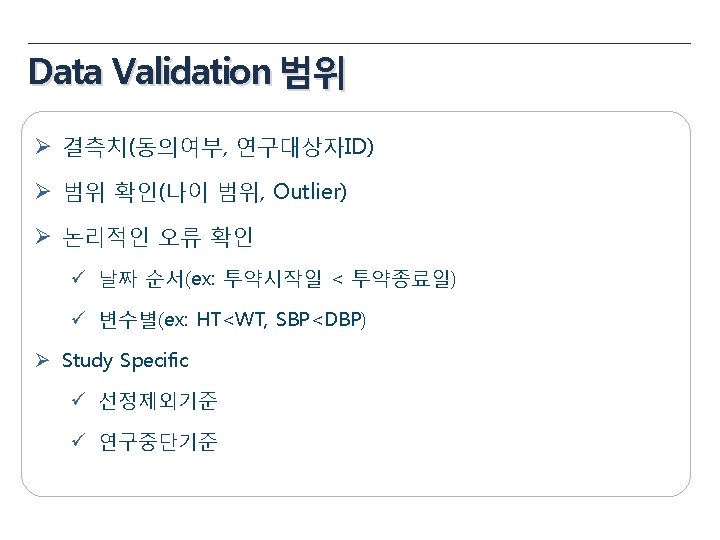
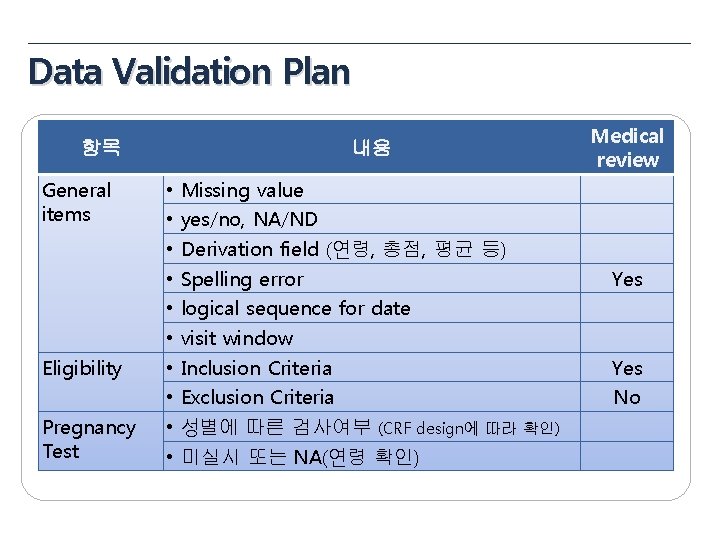
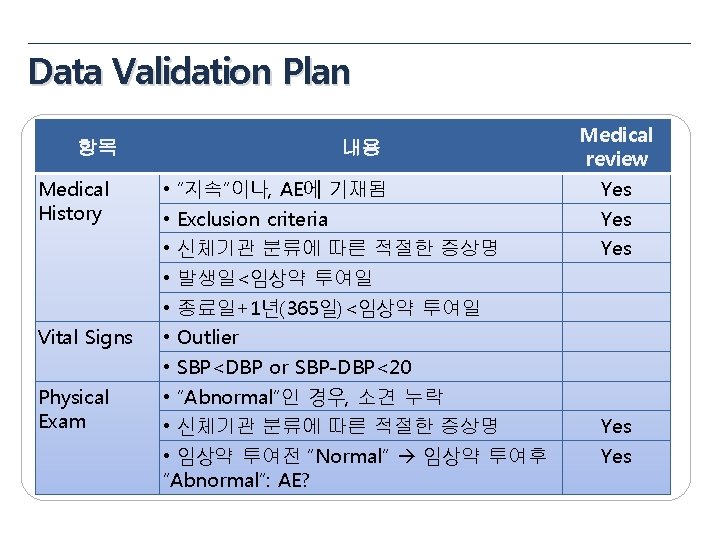
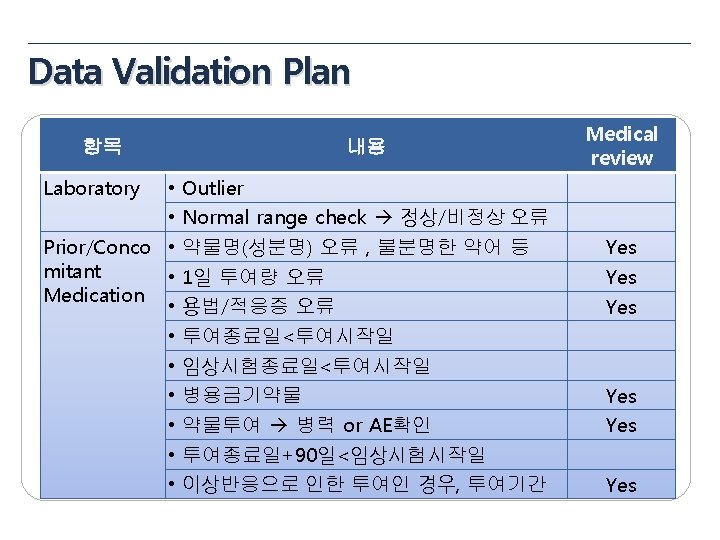
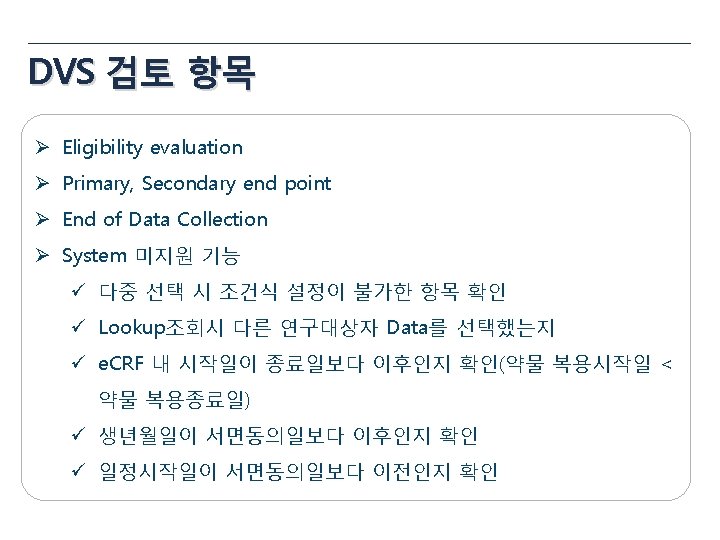
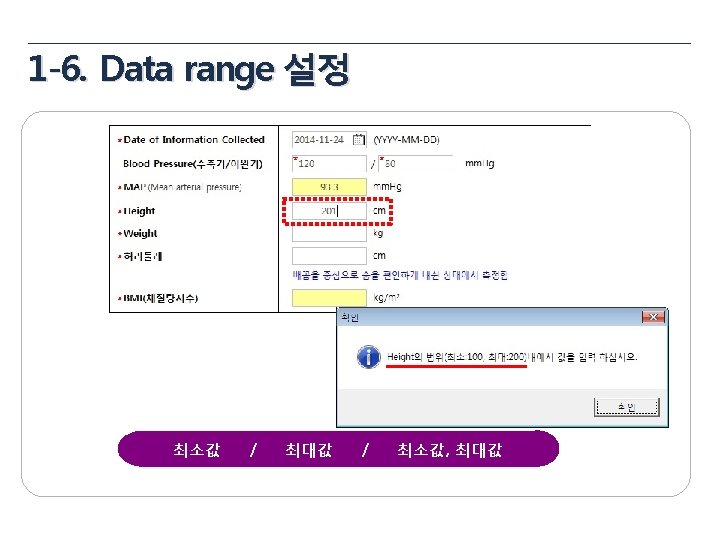
Data Validation Plan 항목 General items Eligibility Pregnancy Test 내용 • • • Missing value yes/no, NA/ND Derivation field (연령, 총점, 평균 등) Spelling error logical sequence for date visit window Inclusion Criteria Exclusion Criteria 성별에 따른 검사여부 (CRF design에 따라 확인) 미실시 또는 NA(연령 확인) Medical review Yes No





Data Validation Specification

Query Flow & Tracking Ø Query Generation & Resolution Process ü 쿼리 양식 작성 ü 쿼리 전달 : email, fax ü 쿼리 해결 기한 내에 해결 요청 ü 쿼리 답변에 따른 DB editing ü 쿼리 Tracking Log ü Data Correction

Data Clarification Form(DCF)

Data Clarification Form(DCF) Resolution

DMP 구성 Ø 종료단계 ü SAE reconciliation ü Data Quality control(QC) ü Database Lock/Un. Lock

SAE reconciliation plan Ø Central SAE DB vs. Study SAE DB : Two DBs with the same data Ø 담당자 Ø 불일치 해결 시점 Ø 불일치 해결 항목 Ø 불일치 해결 절차 Ø 필요시 쿼리 등록


CRF

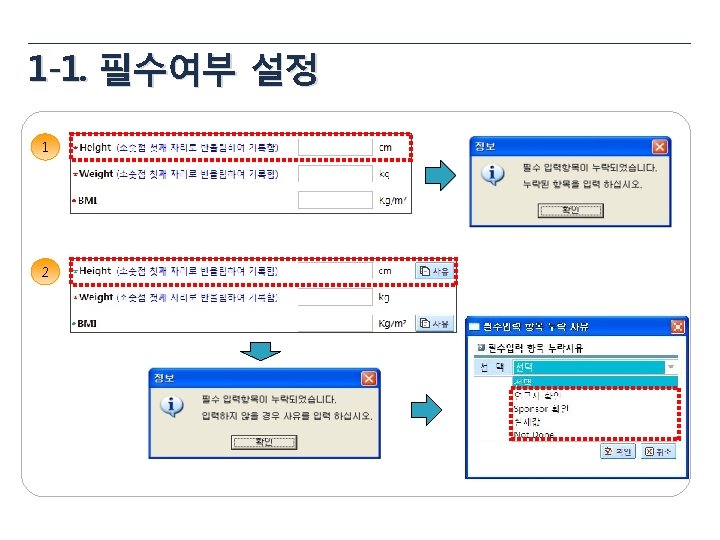
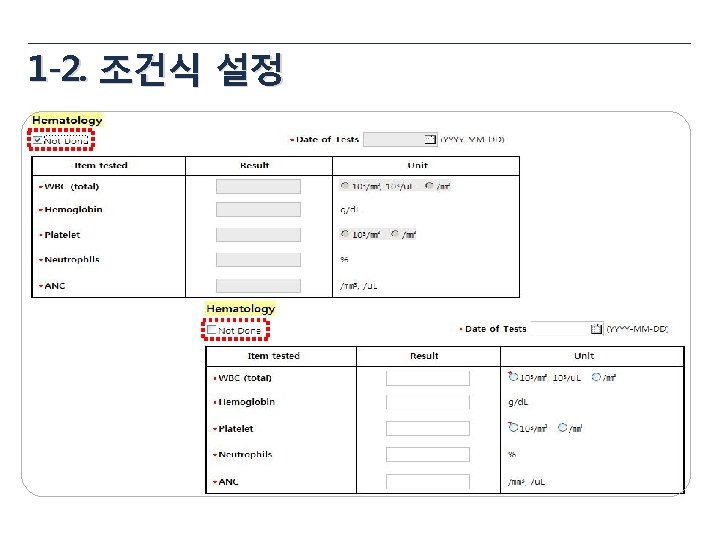
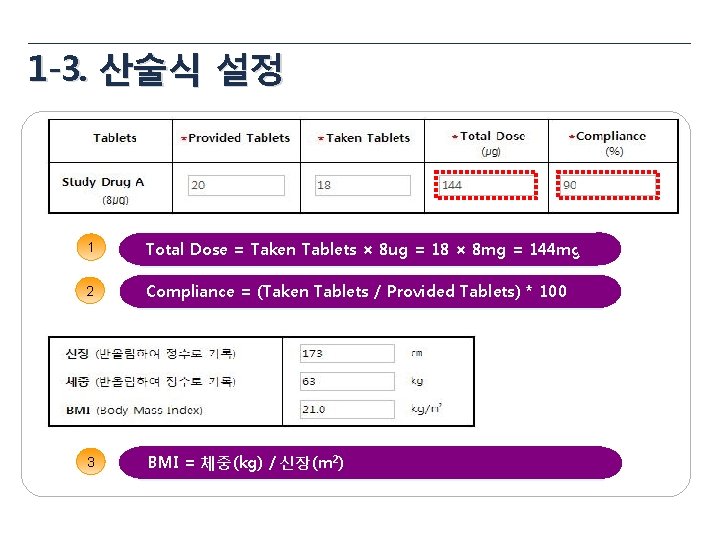
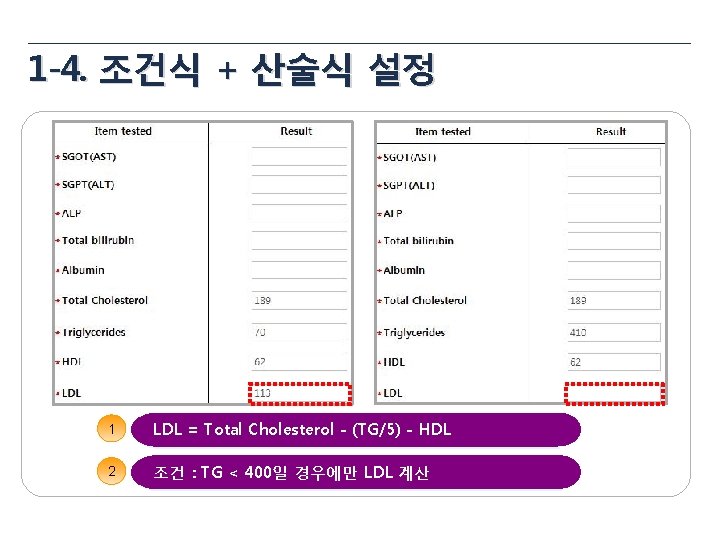
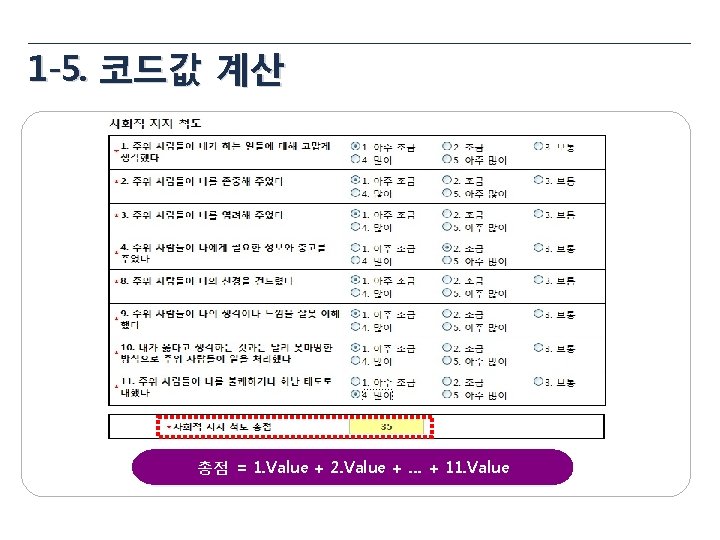
1 -3. 산술식 설정 1 Total Dose = Taken Tablets × 8 ug = 18 × 8 mg = 144 mg 2 Compliance = (Taken Tablets / Provided Tablets) * 100 3 BMI = 체중(kg) / 신장(m 2)
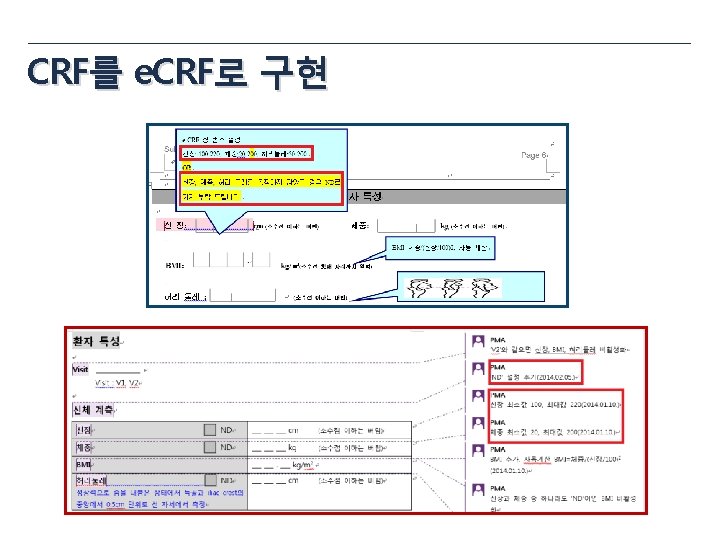

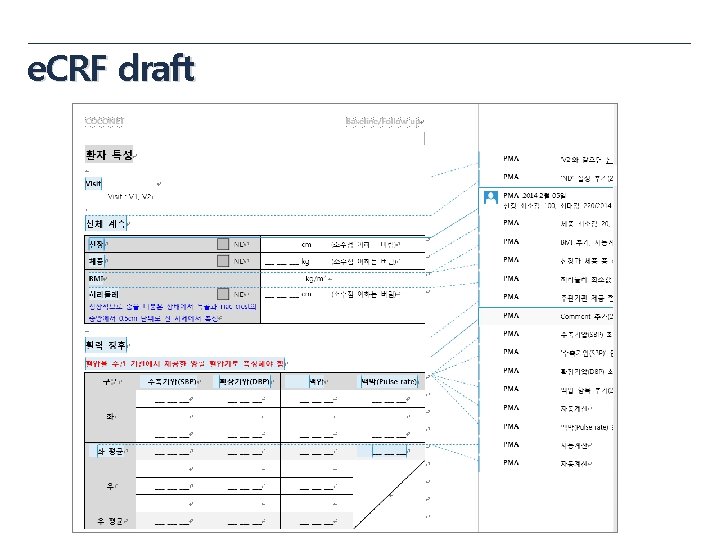
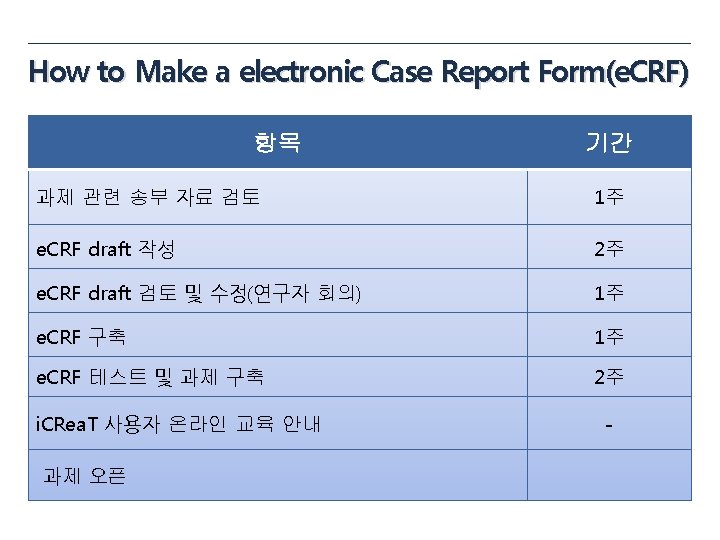
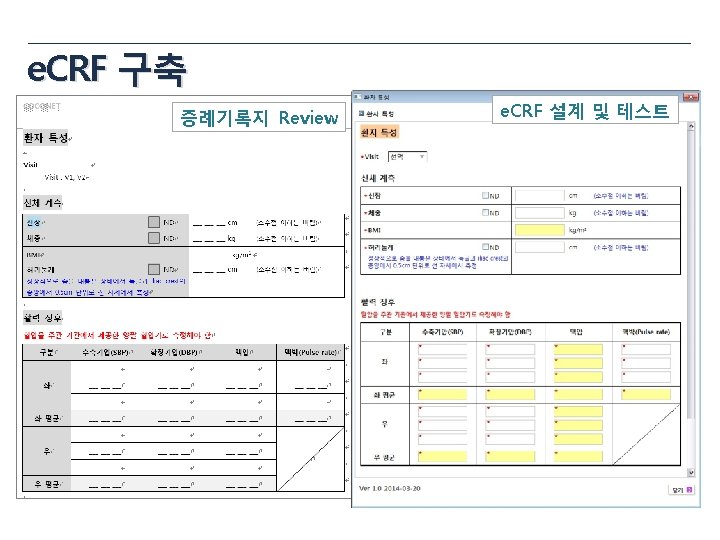
e. CRF draft
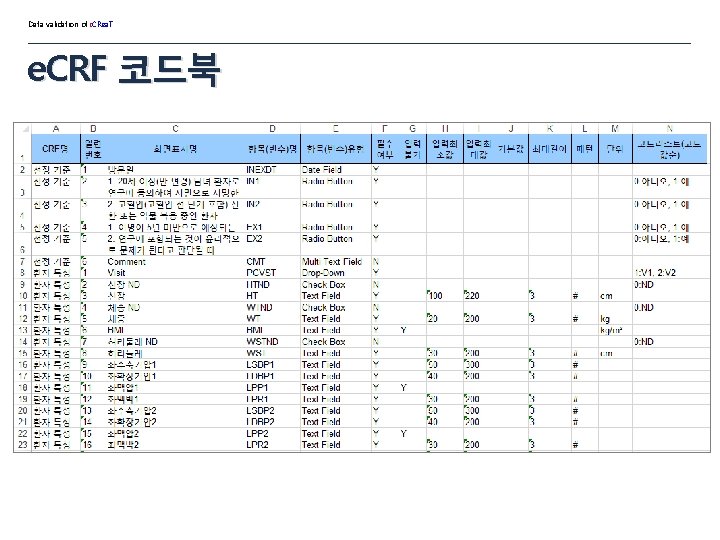
Data validation of i. CRea. T e. CRF 코드북

- Slides: 49