How to name ionic compounds EQ How do



- Slides: 15

How to name ionic compounds EQ: How do we name ionic compounds? Ionic Nomenclature IV

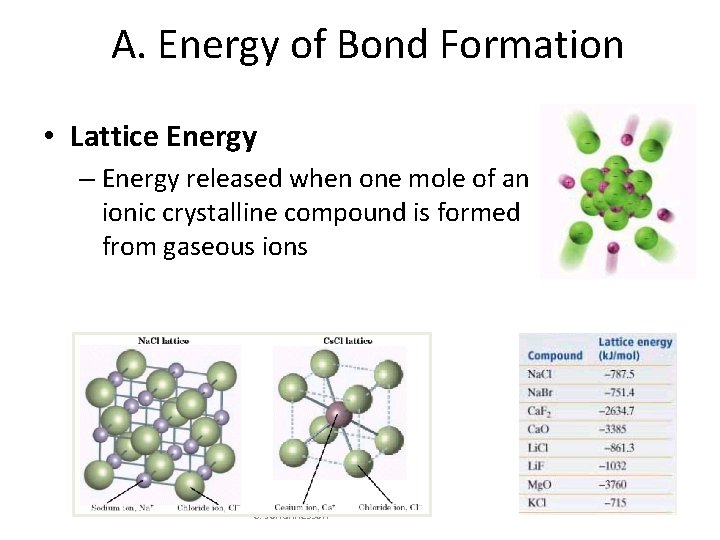
A. Energy of Bond Formation • Lattice Energy – Energy released when one mole of an ionic crystalline compound is formed from gaseous ions C. Johannesson

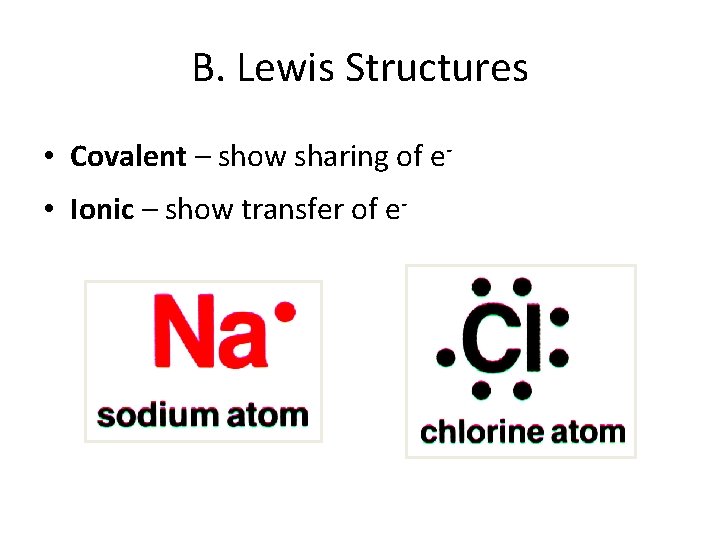
B. Lewis Structures • Covalent – show sharing of e • Ionic – show transfer of e-

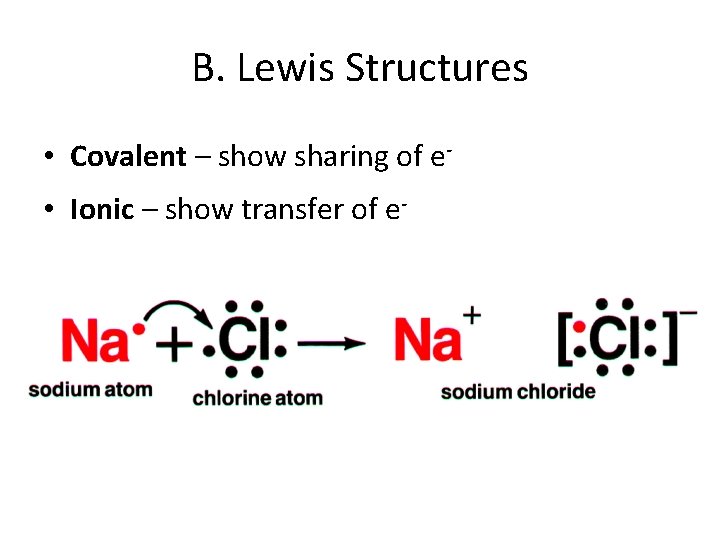
B. Lewis Structures • Covalent – show sharing of e • Ionic – show transfer of e-

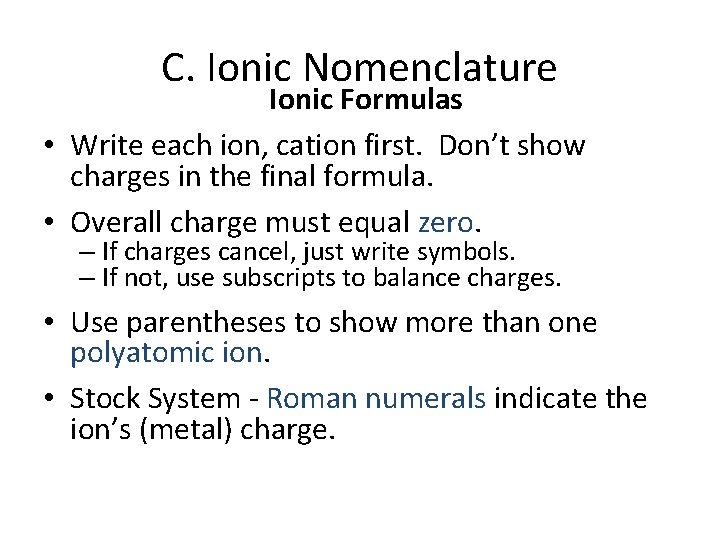
C. Ionic Nomenclature Ionic Formulas • Write each ion, cation first. Don’t show charges in the final formula. • Overall charge must equal zero. – If charges cancel, just write symbols. – If not, use subscripts to balance charges. • Use parentheses to show more than one polyatomic ion. • Stock System - Roman numerals indicate the ion’s (metal) charge.

C. Ionic Nomenclature Ionic Names • Write the names of both ions, cation first. • Change ending of monatomic ions to -ide. • Polyatomic ions have special names. • Stock System - Use Roman numerals to show the ion’s charge if more than one is possible. Overall charge must equal zero.

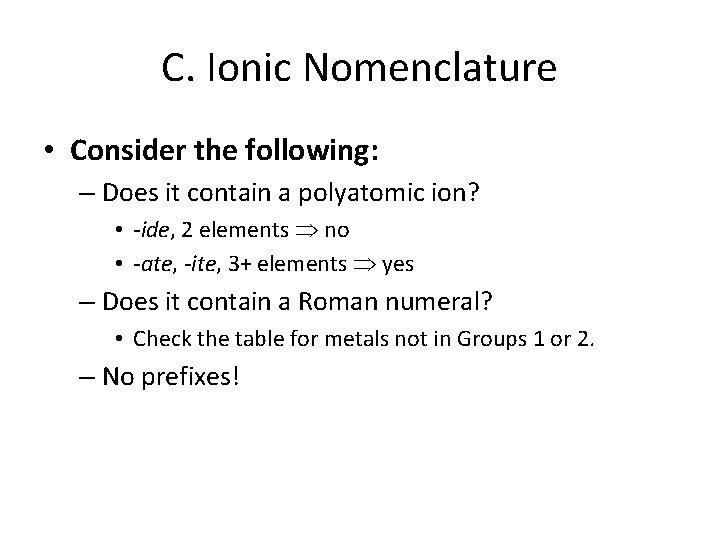
C. Ionic Nomenclature • Consider the following: – Does it contain a polyatomic ion? • -ide, 2 elements no • -ate, -ite, 3+ elements yes – Does it contain a Roman numeral? • Check the table for metals not in Groups 1 or 2. – No prefixes!

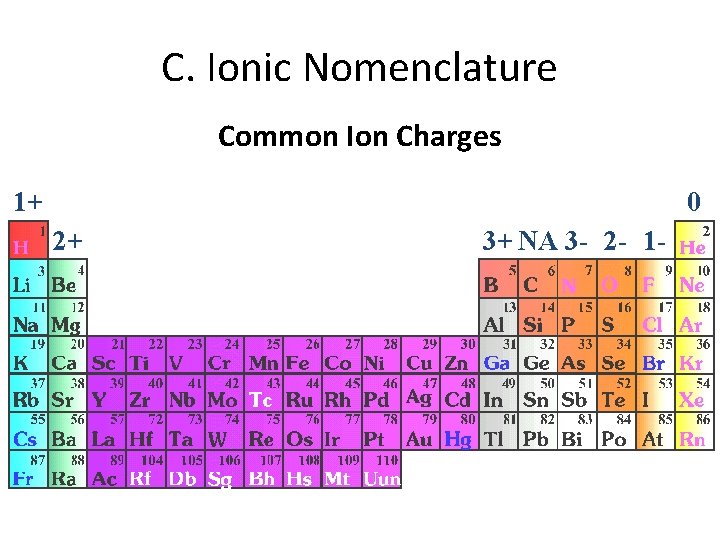
C. Ionic Nomenclature Common Ion Charges 1+ 0 2+ 3+ NA 3 - 2 - 1 -

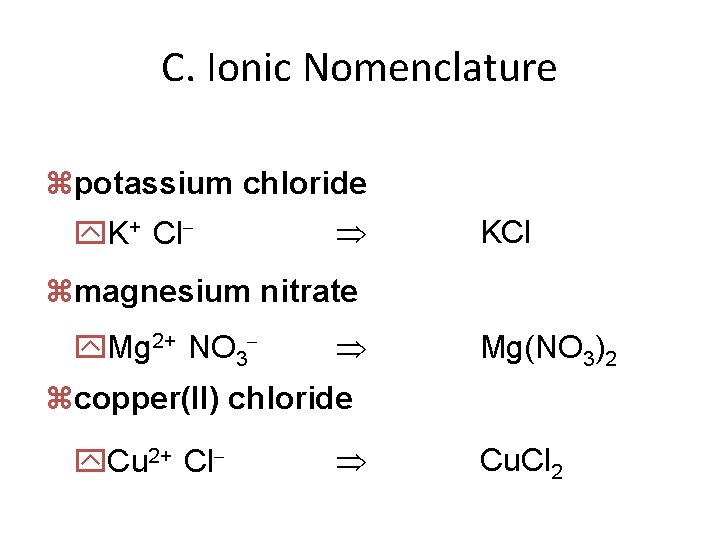
C. Ionic Nomenclature zpotassium chloride y. K+ Cl- KCl zmagnesium nitrate y. Mg 2+ NO 3 - Mg(NO 3)2 zcopper(II) chloride y. Cu 2+ Cl- Cu. Cl 2

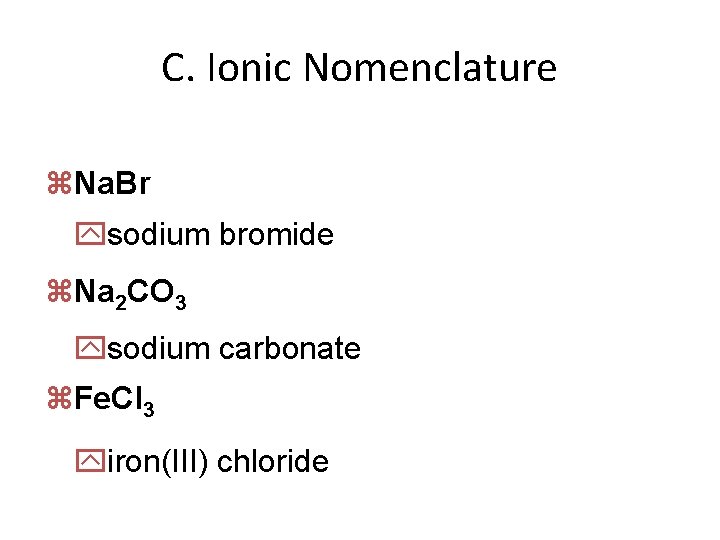
C. Ionic Nomenclature z. Na. Br ysodium bromide z. Na 2 CO 3 ysodium carbonate z. Fe. Cl 3 yiron(III) chloride

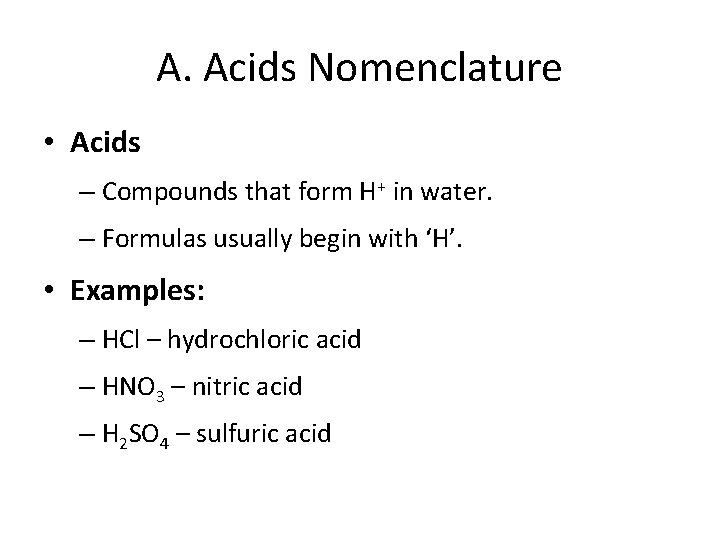
A. Acids Nomenclature • Acids – Compounds that form H+ in water. – Formulas usually begin with ‘H’. • Examples: – HCl – hydrochloric acid – HNO 3 – nitric acid – H 2 SO 4 – sulfuric acid

B. Acid Nomenclature

B. Acid Nomenclature

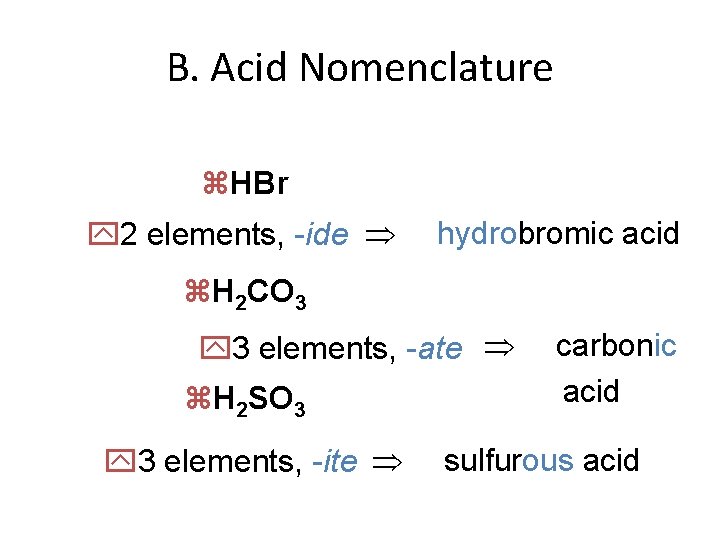
B. Acid Nomenclature z. HBr y 2 elements, -ide hydrobromic acid z. H 2 CO 3 y 3 elements, -ate z. H 2 SO 3 y 3 elements, -ite carbonic acid sulfurous acid

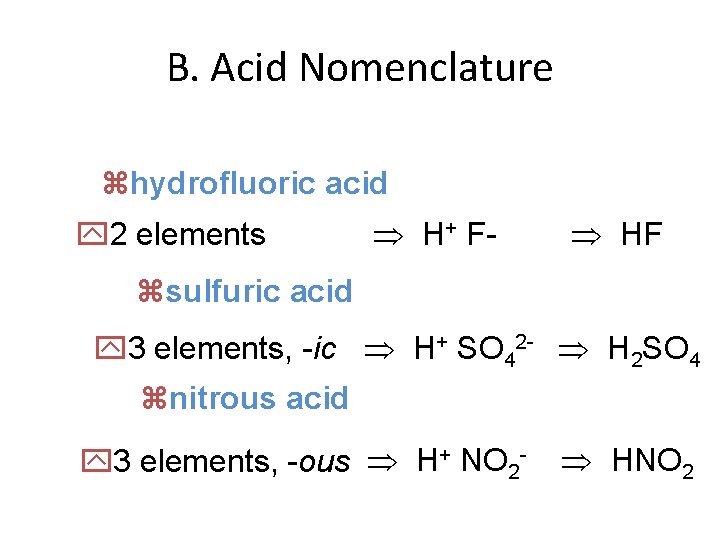
B. Acid Nomenclature zhydrofluoric acid y 2 elements H+ F- HF zsulfuric acid y 3 elements, -ic H+ SO 42 - H 2 SO 4 znitrous acid y 3 elements, -ous H+ NO 2 - HNO 2