How to mix a Standard Solution Zumdahl De


- Slides: 13

How to mix a Standard Solution Zumdahl, De. Coste, World of Chemistry 2002, page 480

Process of Making a Standard Solution from Liquids Zumdahl, De. Coste, World of Chemistry 2002, page 483

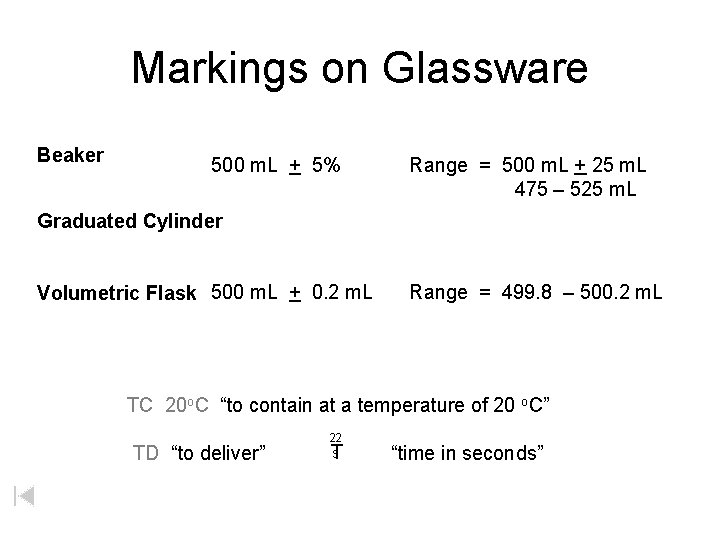
Markings on Glassware Beaker 500 m. L + 5% Range = 500 m. L + 25 m. L 475 – 525 m. L Graduated Cylinder Volumetric Flask 500 m. L + 0. 2 m. L Range = 499. 8 – 500. 2 m. L TC 20 o. C “to contain at a temperature of 20 o. C” TD “to deliver” 22 s T “time in seconds”

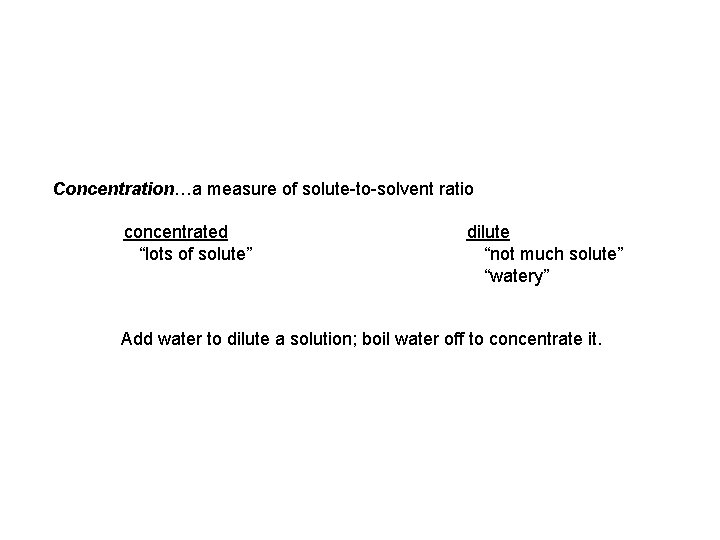
Concentration…a measure of solute-to-solvent ratio concentrated “lots of solute” dilute “not much solute” “watery” Add water to dilute a solution; boil water off to concentrate it.

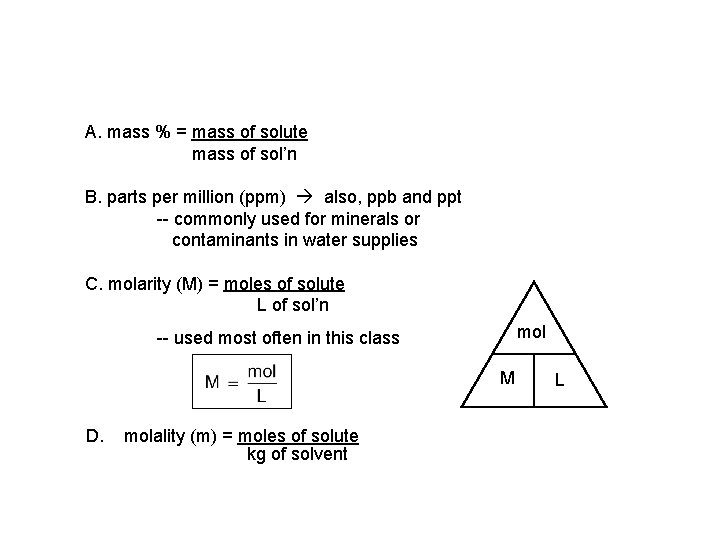
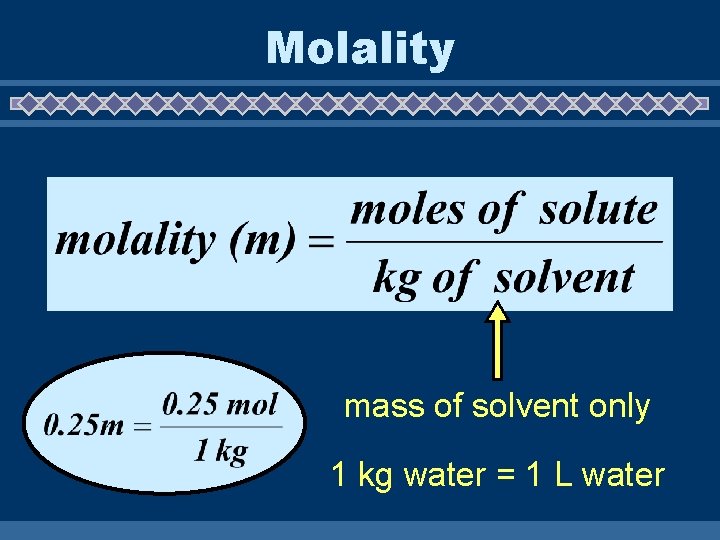
A. mass % = mass of solute mass of sol’n B. parts per million (ppm) also, ppb and ppt -- commonly used for minerals or contaminants in water supplies C. molarity (M) = moles of solute L of sol’n mol -- used most often in this class M D. molality (m) = moles of solute kg of solvent L

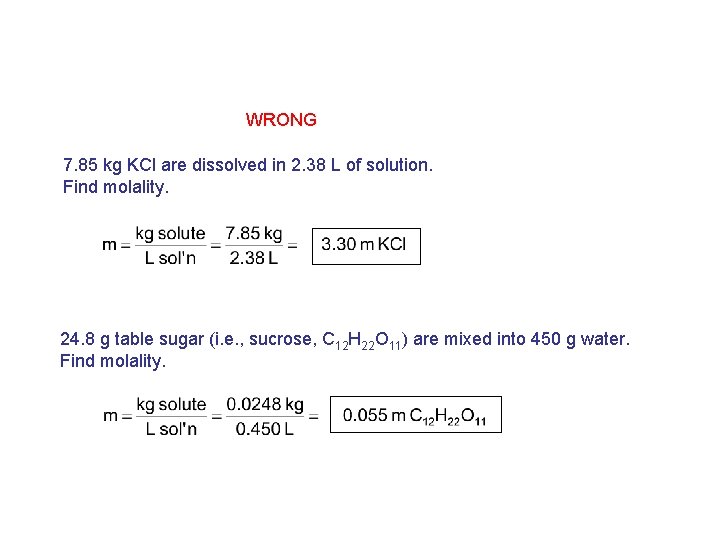
WRONG 7. 85 kg KCl are dissolved in 2. 38 L of solution. Find molality. 24. 8 g table sugar (i. e. , sucrose, C 12 H 22 O 11) are mixed into 450 g water. Find molality.

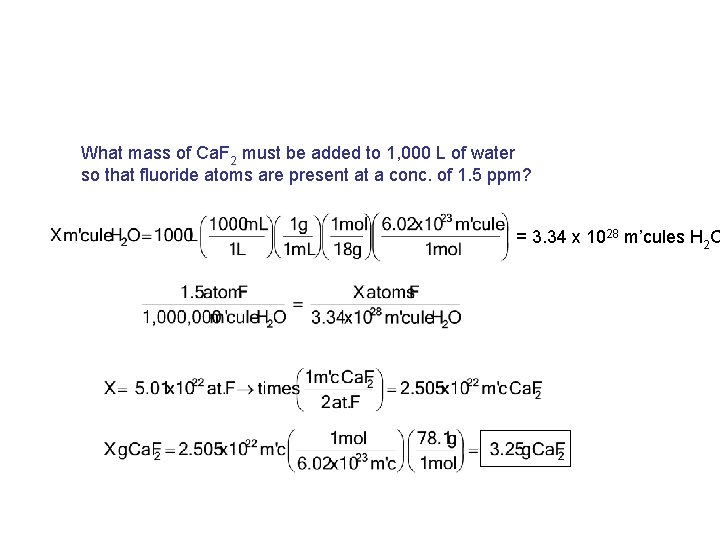
What mass of Ca. F 2 must be added to 1, 000 L of water so that fluoride atoms are present at a conc. of 1. 5 ppm? = 3. 34 x 1028 m’cules H 2 O

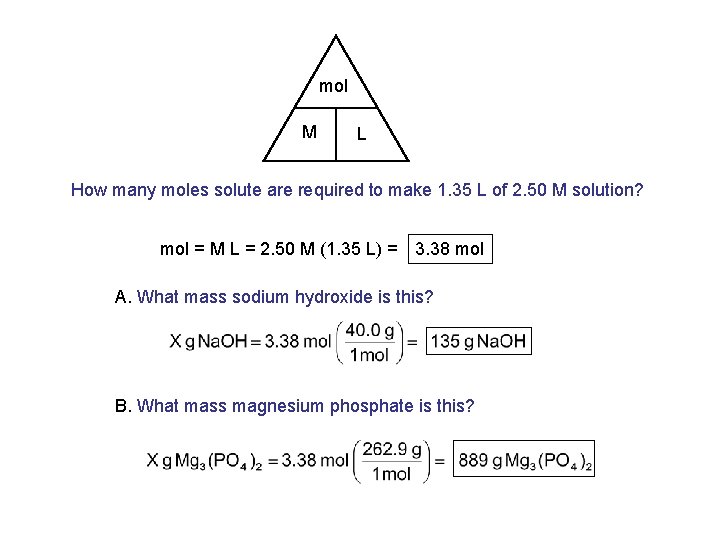
mol M L How many moles solute are required to make 1. 35 L of 2. 50 M solution? mol = M L = 2. 50 M (1. 35 L) = 3. 38 mol A. What mass sodium hydroxide is this? B. What mass magnesium phosphate is this?

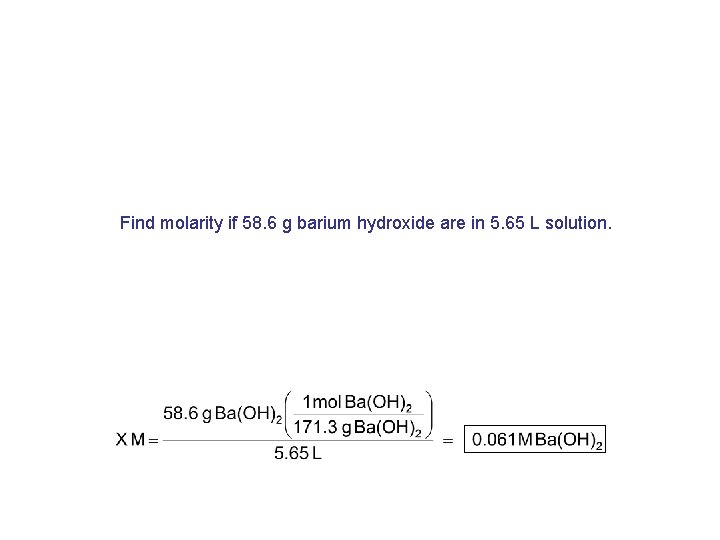
Find molarity if 58. 6 g barium hydroxide are in 5. 65 L solution.

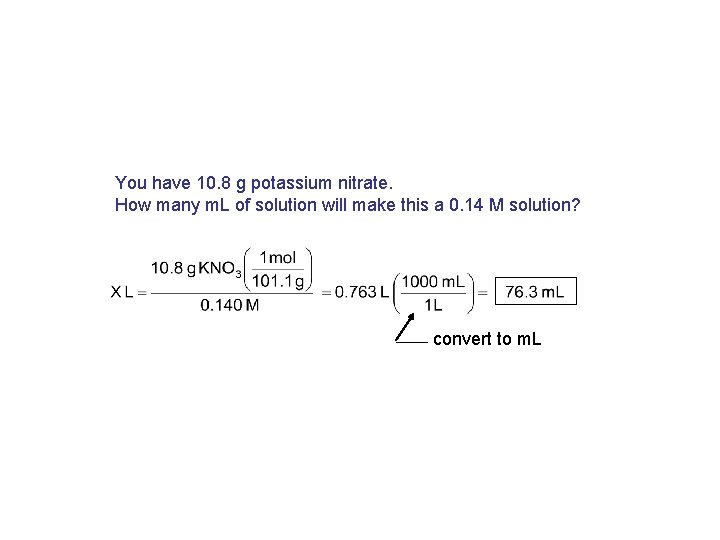
You have 10. 8 g potassium nitrate. How many m. L of solution will make this a 0. 14 M solution? convert to m. L

Concentration u The amount of solute in a solution. u Describing Concentration • % by mass - medicated creams • % by volume - rubbing alcohol • ppm, ppb - water contaminants • molarity - used by chemists • molality - used by chemists

Molality mass of solvent only 1 kg water = 1 L water

Molarity of Solutions http: //www. unit 5. org/christjs/temp T 27 d. Fields-Jeff/Solutions 1. htm