How to handle samples for coagulation analyses AnneMette






- Slides: 13

How to handle samples for coagulation analyses Anne-Mette Hvas Department of Clinical Biochemistry Center for Haemophilia and Thrombosis Aarhus University Hospital, Skejby Denmark 1 >>

For the next 15 minutes ¡ Preparation of the patient ¡ Specimen collection l l l Venipuncture Vascular access device Tubes and anticoagulant ¡ Specimen transport and processing ¡ Sample Storage 2 >>

Preparation of the patient – the ideal Standardized time of the day Overnight fast or a light meal No alchohol during the past 18 -24 h No coffee during the past 1 h No smoking during the past 1 h 20 minutes rest before blood sampling Specified posture (sitting/lying) 3 >>

Preparation of the patient – possible and acceptable Routine laboratory PT/INR, APTT No preparation of the patient 4 >>

Preparation of the patient – possible and acceptable Routine laboratory PT/INR, APTT No preparation of the patient Specialised coagulation laboratory • Coagulation factors, natural anticoagulants, von Willebrand factor No preparation of the patient • Markers of fibrinolysis Standardized time of the day Tissue-type plasminogen activator (t-PA) Overnight fast or a light meal Plasminogen activator inhibitor (PAI-1) No alchohol during the past 18 -24 h Plasminogen activity No coffee during the past 1 h Plasmin-inhibitor No smoking during the past 1 h Specified posture (sitting/lying) 20 minutes rest before blood sampling 5 >>

Specimen collection - what is the problem? As soon as the blood vessel is entered: ¡ Tissue factor is released activation of extrinsic pathway ¡ Platelets are activated ¡ Coagulation factors are activated ¡ Natural anticoagulants are activated 6 >>

Specimen collection ¡ Venipuncture l Needle gauge from 21 to 19 l Max 25 m. L (20 -gauge needle), max 50 m. L (19 -gauge needle) l Torniquet application for max 1 min with minimal stasis l Collected directly into the tube containing the anticoagulant l Discard the first 5 m. L collected (except for PT/INR or APTT) l The tube must be correctly filled 7 >>

Specimen collection ¡ Vascular access device l Avoid air leaks l Avoid heparin contamination and specimen dilution ¡ Flush the line with 5 m. L saline and discard the first 5 m. L or six dead space volumes 8 >>

Specimen collection ¡ Tubes and anticoagulant l Tubes of non-reactive materials (polypropylene or siliconized glass) l 3. 2% trisodium citrate l The proportion of blood: trisodium citrate are usually 9: 1 9 >>

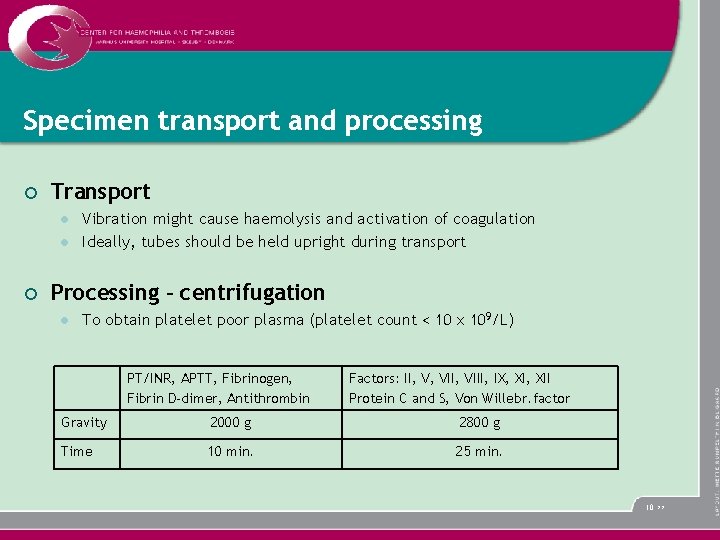
Specimen transport and processing ¡ Transport l l ¡ Vibration might cause haemolysis and activation of coagulation Ideally, tubes should be held upright during transport Processing – centrifugation l To obtain platelet poor plasma (platelet count < 10 x 109/L) PT/INR, APTT, Fibrinogen, Fibrin D-dimer, Antithrombin Factors: II, V, VIII, IX, XII Protein C and S, Von Willebr. factor Gravity 2000 g 2800 g Time 10 min. 25 min. 10 >>

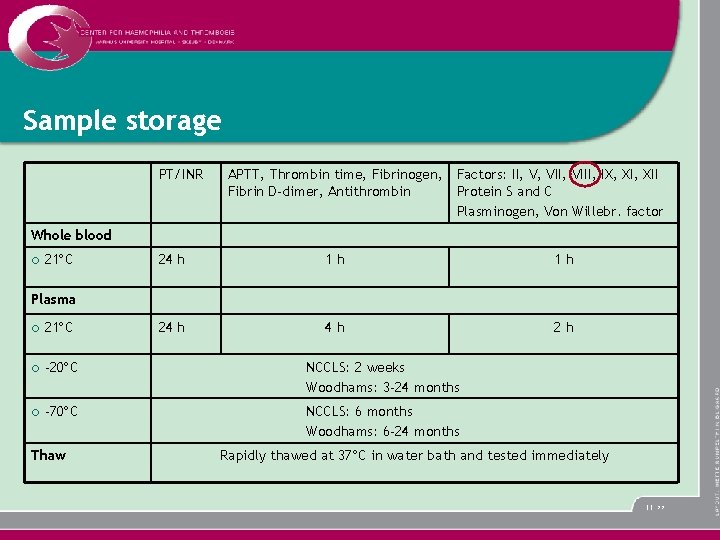
Sample storage PT/INR APTT, Thrombin time, Fibrinogen, Factors: II, V, VIII, IX, XII Fibrin D-dimer, Antithrombin Protein S and C Plasminogen, Von Willebr. factor Whole blood ¡ 21 C 24 h 1 h 1 h 24 h 4 h 2 h Plasma ¡ 21 C ¡ -20 C NCCLS: 2 weeks Woodhams: 3 -24 months ¡ -70 C NCCLS: 6 months Woodhams: 6 -24 months Thaw Rapidly thawed at 37 C in water bath and tested immediately 11 >>

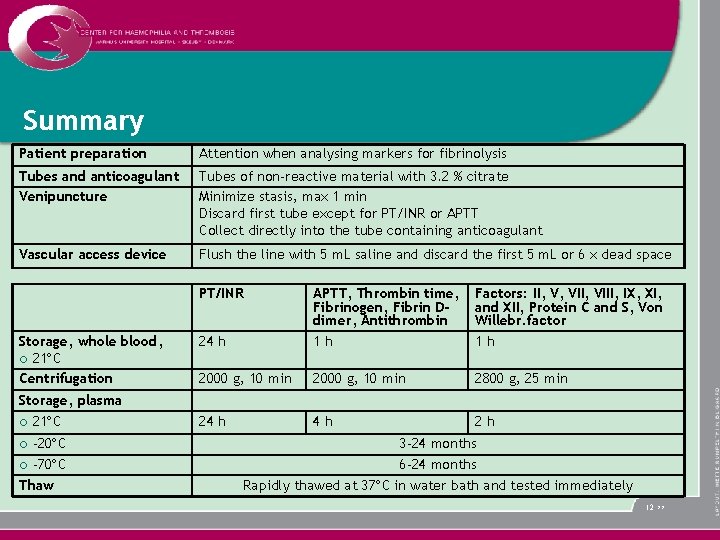
Summary Patient preparation Attention when analysing markers for fibrinolysis Tubes and anticoagulant Venipuncture Tubes of non-reactive material with 3. 2 % citrate Minimize stasis, max 1 min Discard first tube except for PT/INR or APTT Collect directly into the tube containing anticoagulant Vascular access device Flush the line with 5 m. L saline and discard the first 5 m. L or 6 x dead space PT/INR Storage, whole blood, ¡ 21 C Centrifugation Storage, plasma ¡ 21 C -20 C ¡ -70 C Thaw ¡ 24 h APTT, Thrombin time, Fibrinogen, Fibrin Ddimer, Antithrombin 1 h Factors: II, V, VIII, IX, XI, and XII, Protein C and S, Von Willebr. factor 1 h 2000 g, 10 min 2800 g, 25 min 24 h 4 h 2 h 3 -24 months 6 -24 months Rapidly thawed at 37 C in water bath and tested immediately 12 >>

Main documentation ¡ National Committee for Clinical Laboratory Standards. Fourth edition 2003. Document H 21 -A 3 ¡ Woodhams et al. Blood Coagulation and Fibrinolysis 2001; 12: 229 -236 ¡ Laboratory Techniques in Thrombosis. A Manual. 2 nd revised edition of ECAT Assay Procedures Edited by J Jespersen, RM Bertina and F Haverkate 13 >>