How to Draw Lewis Structures Lewis Structures 1




- Slides: 13

How to Draw Lewis Structures

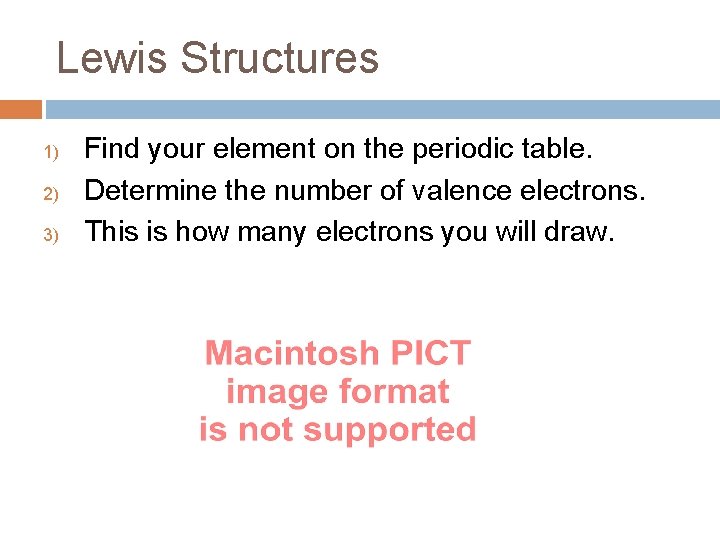
Lewis Structures 1) 2) 3) Find your element on the periodic table. Determine the number of valence electrons. This is how many electrons you will draw.

Lewis Structures • • • Find out which group (column) your element is in. This will tell you the number of valence electrons your element has. You will only draw the valence electrons.

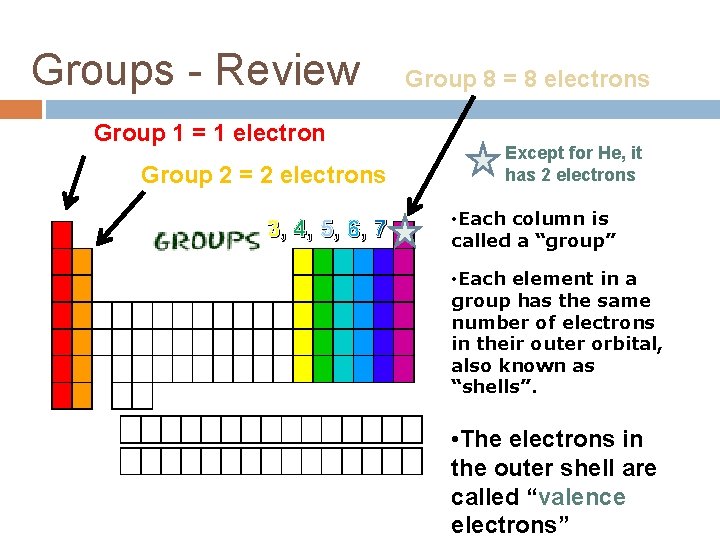
Groups - Review Group 1 = 1 electron Group 2 = 2 electrons 3, 4, 5, 6, 7 Group 8 = 8 electrons Except for He, it has 2 electrons • Each column is called a “group” • Each element in a group has the same number of electrons in their outer orbital, also known as “shells”. • The electrons in the outer shell are called “valence electrons”

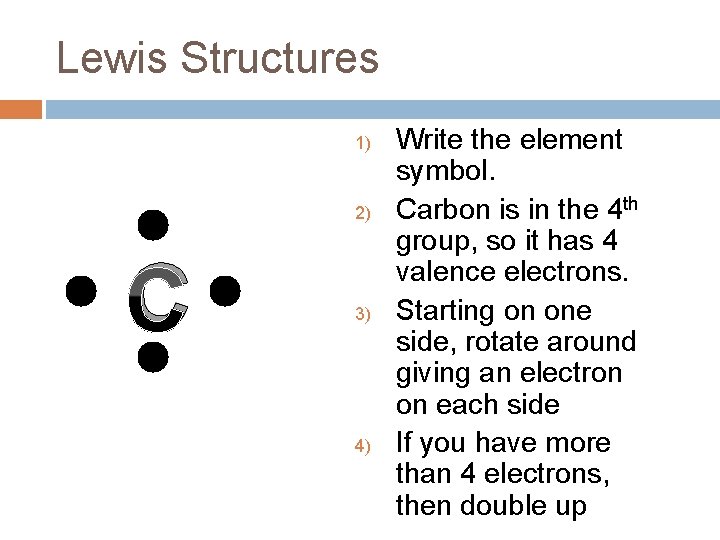
Lewis Structures 1) 2) C 3) 4) Write the element symbol. Carbon is in the 4 th group, so it has 4 valence electrons. Starting on one side, rotate around giving an electron on each side If you have more than 4 electrons, then double up

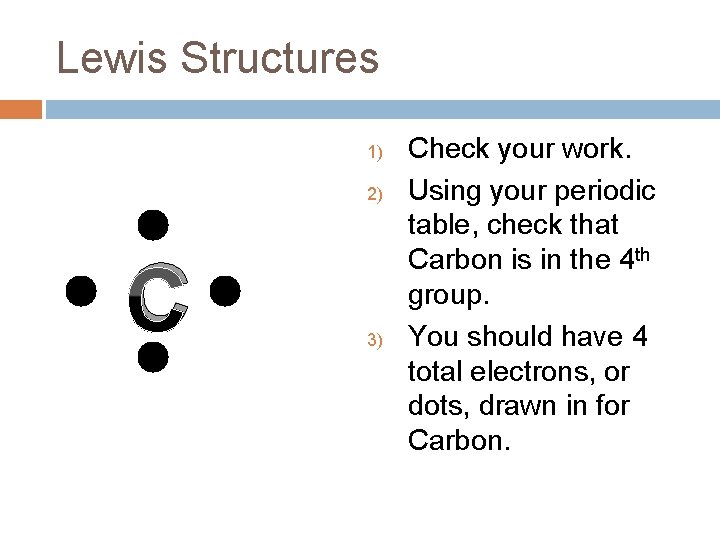
Lewis Structures 1) 2) C 3) Check your work. Using your periodic table, check that Carbon is in the 4 th group. You should have 4 total electrons, or dots, drawn in for Carbon.

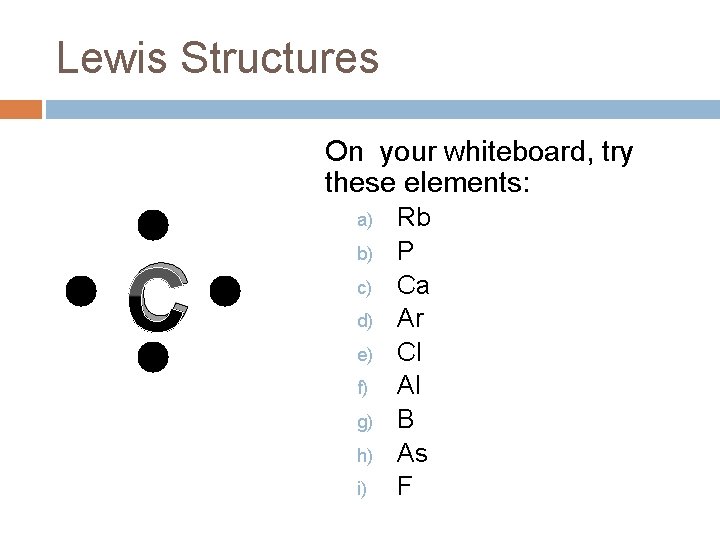
Lewis Structures On your whiteboard, try these elements: a) C b) c) d) e) f) g) h) i) Rb P Ca Ar Cl Al B As F

Forming Ions What is an ion? � An electrically charged particle formed when an atom gains or looses an electron � Loosing electrons form positively charged ions called cations � Gaining electrons form negatively charged ions called anions

Forming ions

Forming Ions The octet rule � Atoms are most stable when they have full valence shells � Gain or loose electrons to have 8 or 0 valence electrons � Will gain or loose …. Which ever involves the fewest electrons moving

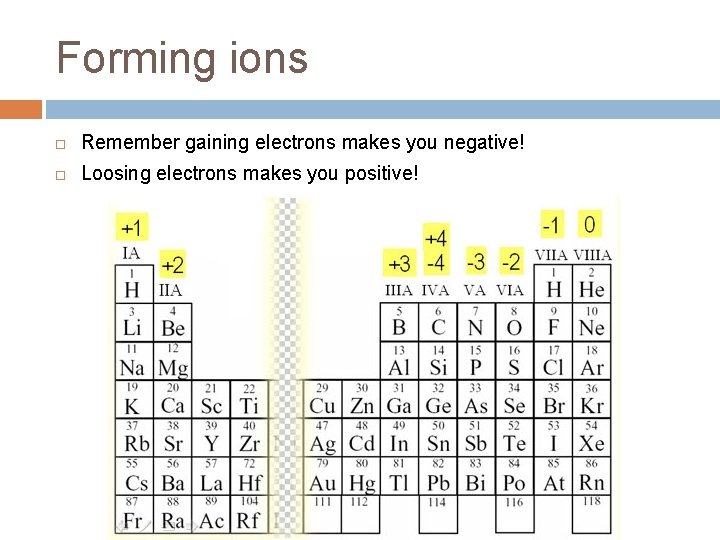
Forming ions Remember gaining electrons makes you negative! Loosing electrons makes you positive!

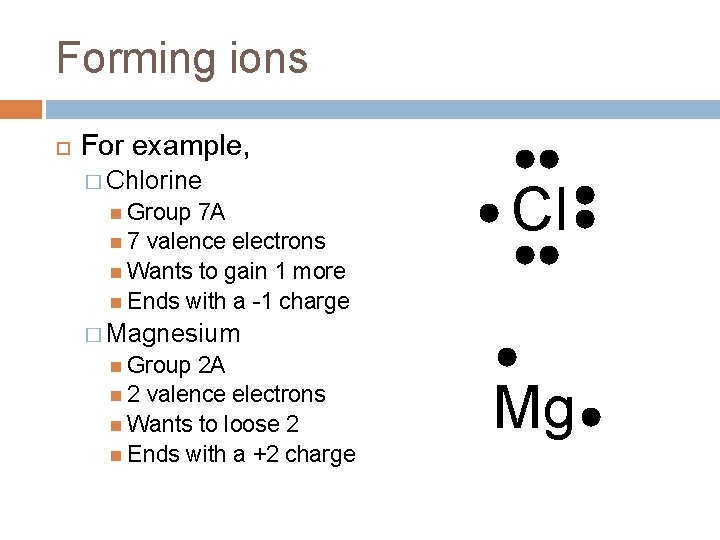
Forming ions For example, � Chlorine Group 7 A 7 valence electrons Wants to gain 1 more Ends with a -1 charge Cl � Magnesium Group 2 A 2 valence electrons Wants to loose 2 Ends with a +2 charge Mg

Forming ions On your whiteboard, try these elements: a) b) c) d) e) f) g) h) i) Rb P Ca Ar Cl Al B As F