How to Draw Bohr Diagrams Calcium Bohr Diagram

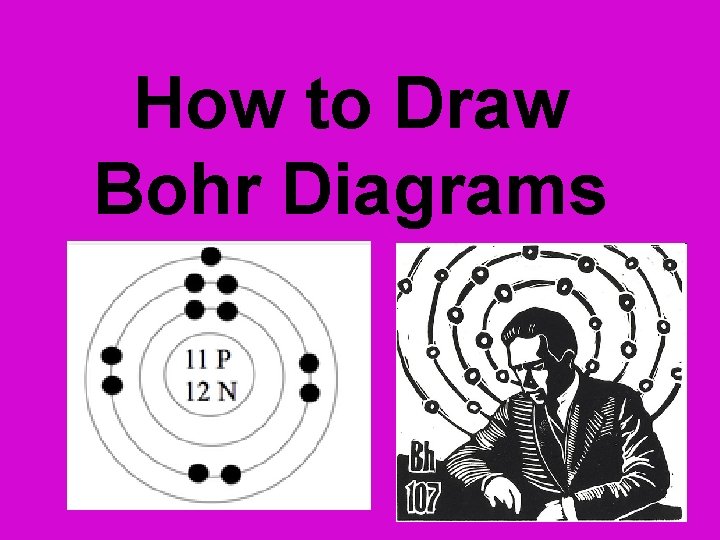
How to Draw Bohr Diagrams
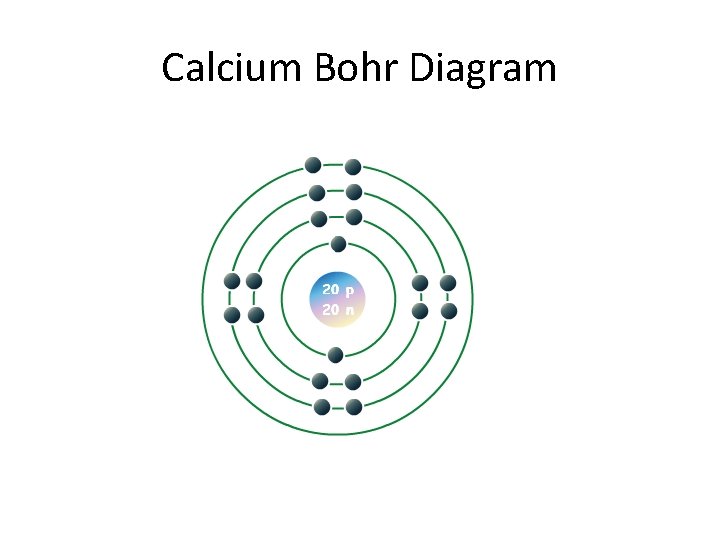
Calcium Bohr Diagram
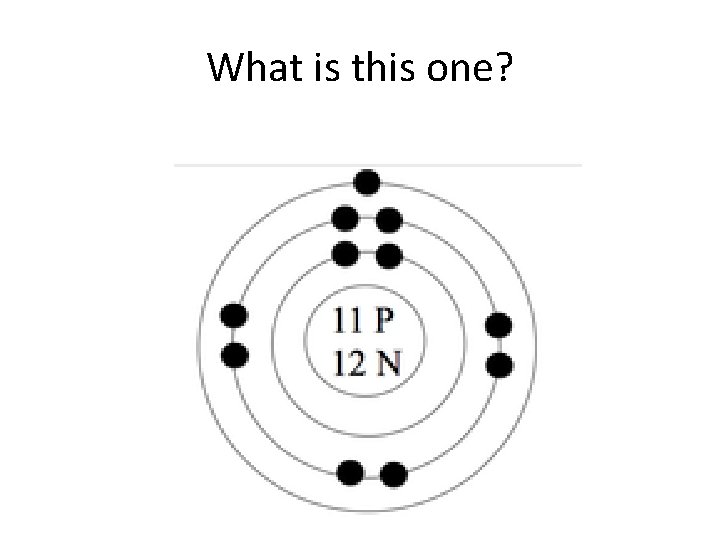
What is this one?
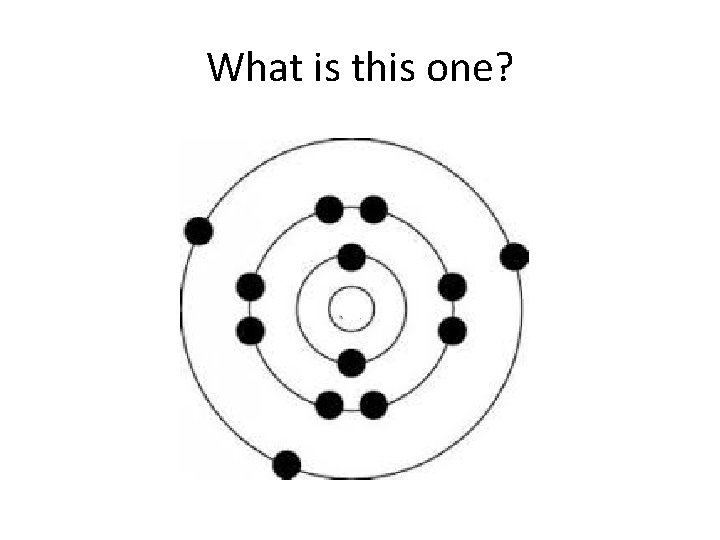
What is this one?
Atomic Structure Review • Atoms have a nucleus that contains Protons and Neutrons • Electrons are contained in shells that surround the nucleus • An atom is made of mostly empty space • Protons have a positive (+) charge • Electrons have a negative (-) charge • Neutrons are Neutral
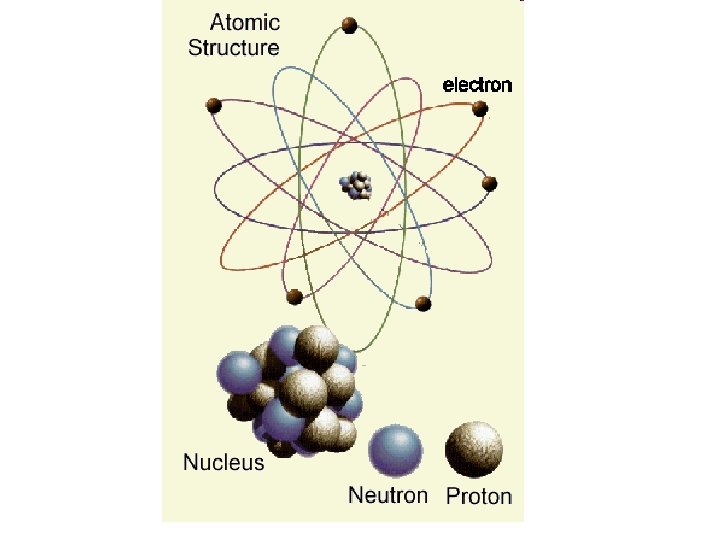
Electrons • Each electron shell can hold a certain number of electrons • Noble Gases have full outer electron shells • All other elements have partially filled outer electron shells Electron Shell 1 K Number of Electrons 2 2 L 8 3 M 18 (max) There are others – we are only concerned with the first 20 elements… after that it gets more complicated – you will learn electron configuration in chemistry.

Valence Electrons • The electrons in the outer most electron shell are called valence electrons • The shell containing electrons that is furthest from the nucleus is called the valence shell • The number of electron shells with electrons is the same as (=) the period number • Atoms will try to gain or lose electrons to have a full valence shell
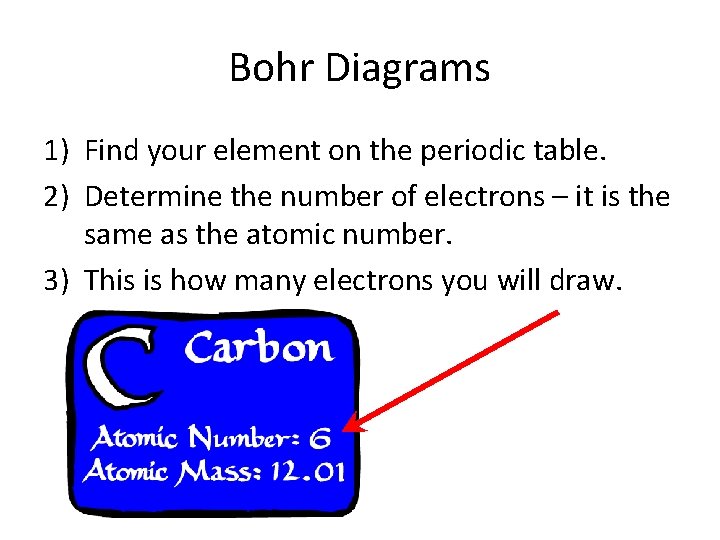
Bohr Diagrams 1) Find your element on the periodic table. 2) Determine the number of electrons – it is the same as the atomic number. 3) This is how many electrons you will draw.
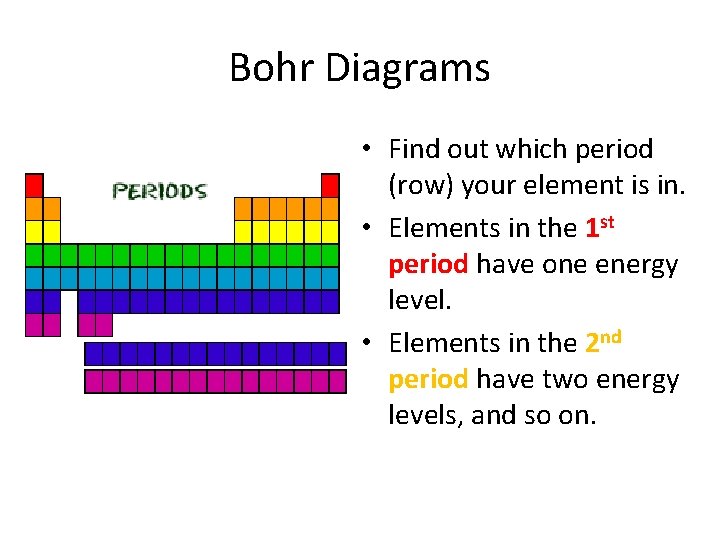
Bohr Diagrams • Find out which period (row) your element is in. • Elements in the 1 st period have one energy level. • Elements in the 2 nd period have two energy levels, and so on.
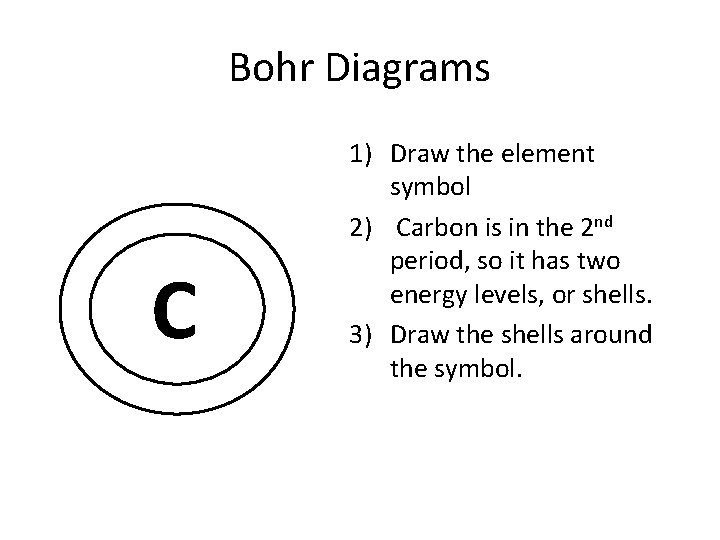
Bohr Diagrams C 1) Draw the element symbol 2) Carbon is in the 2 nd period, so it has two energy levels, or shells. 3) Draw the shells around the symbol.
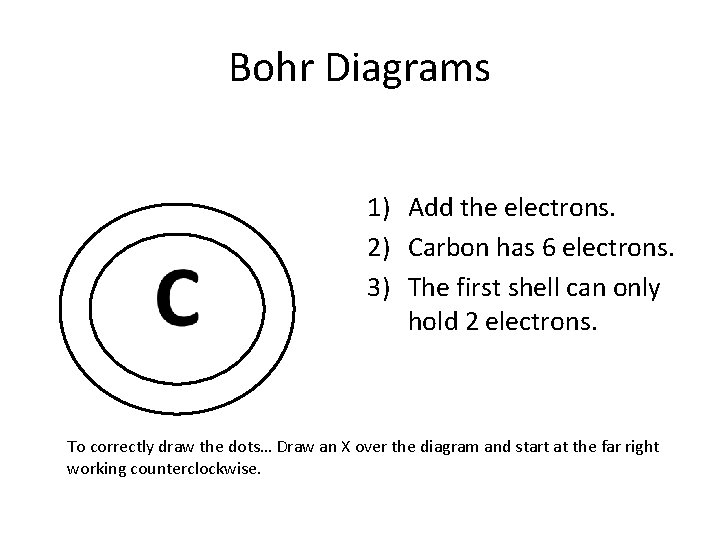
Bohr Diagrams 1) Add the electrons. 2) Carbon has 6 electrons. 3) The first shell can only hold 2 electrons. To correctly draw the dots… Draw an X over the diagram and start at the far right working counterclockwise.
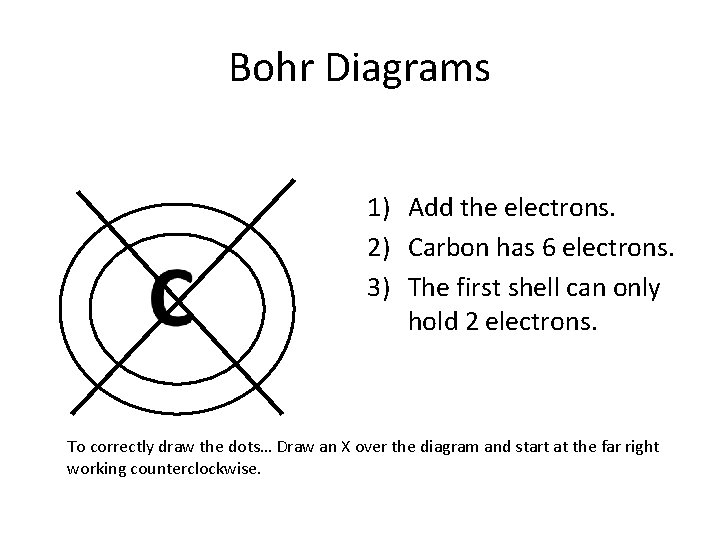
Bohr Diagrams 1) Add the electrons. 2) Carbon has 6 electrons. 3) The first shell can only hold 2 electrons. To correctly draw the dots… Draw an X over the diagram and start at the far right working counterclockwise.
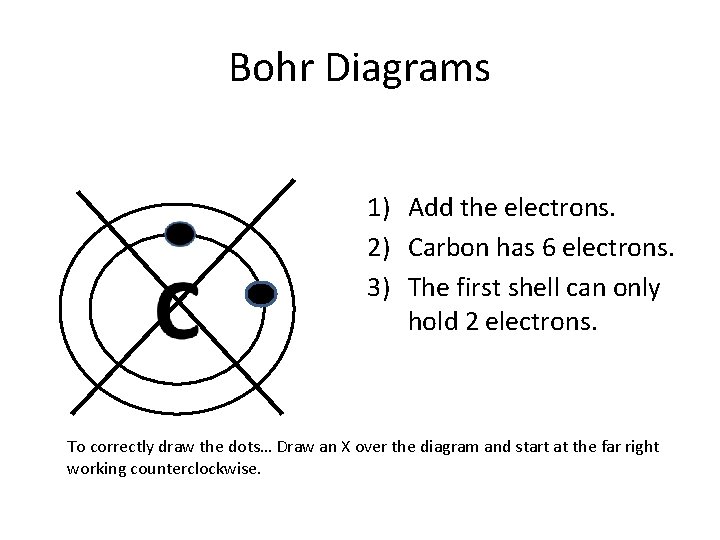
Bohr Diagrams 1) Add the electrons. 2) Carbon has 6 electrons. 3) The first shell can only hold 2 electrons. To correctly draw the dots… Draw an X over the diagram and start at the far right working counterclockwise.
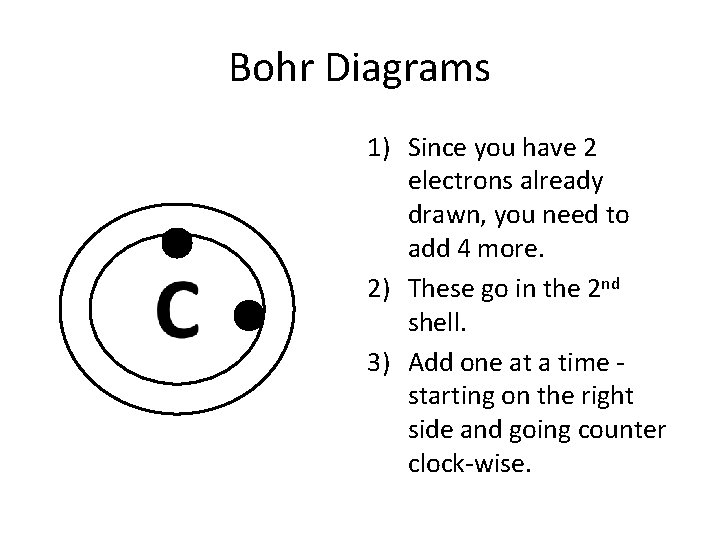
Bohr Diagrams 1) Since you have 2 electrons already drawn, you need to add 4 more. 2) These go in the 2 nd shell. 3) Add one at a time starting on the right side and going counter clock-wise.
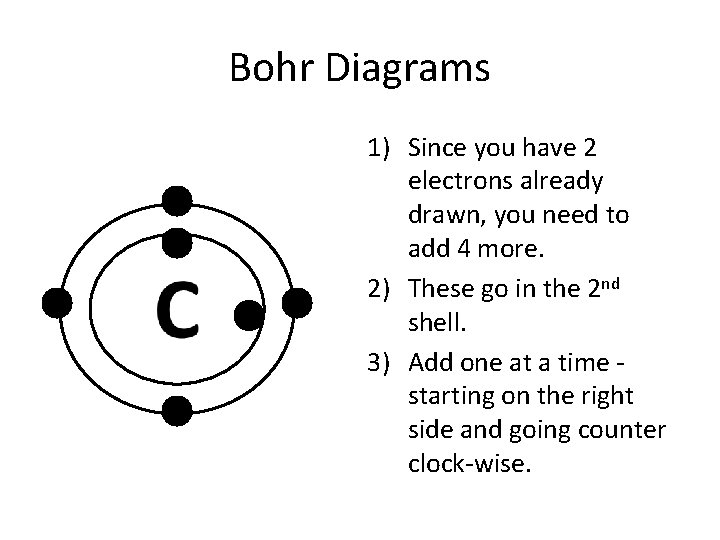
Bohr Diagrams 1) Since you have 2 electrons already drawn, you need to add 4 more. 2) These go in the 2 nd shell. 3) Add one at a time starting on the right side and going counter clock-wise.
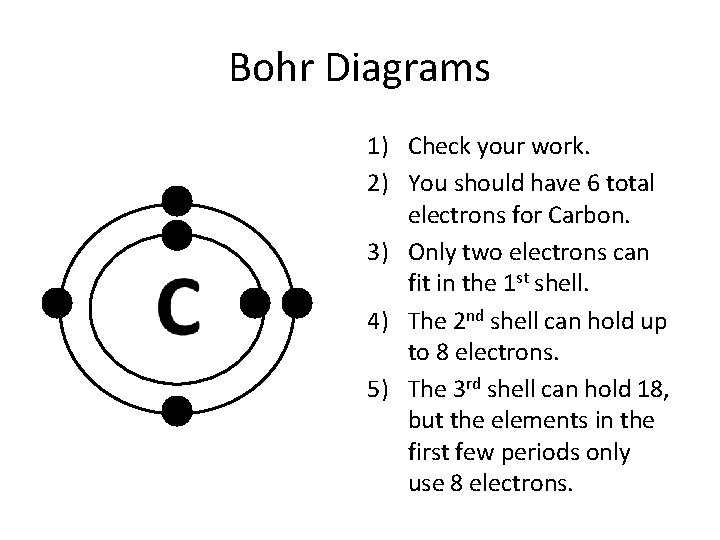
Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1 st shell. 4) The 2 nd shell can hold up to 8 electrons. 5) The 3 rd shell can hold 18, but the elements in the first few periods only use 8 electrons.
• Let’s do Find it on periodic table… how many shells? How many VALENCE electrons? BOHR DIAGRAM Atomic # (which means #Protons) Electrons (matches the # of Protons) K -First Shell L -Second Shell M -Third Shell Lewis Dot Structure
LEWIS STRUCTURES • Another way to draw elements using VALENCE ELECTRONS
Lewis Structures • Find out which group (column) your element is in. • This will tell you the number of valence electrons your element has. • You will only draw the valence electrons.
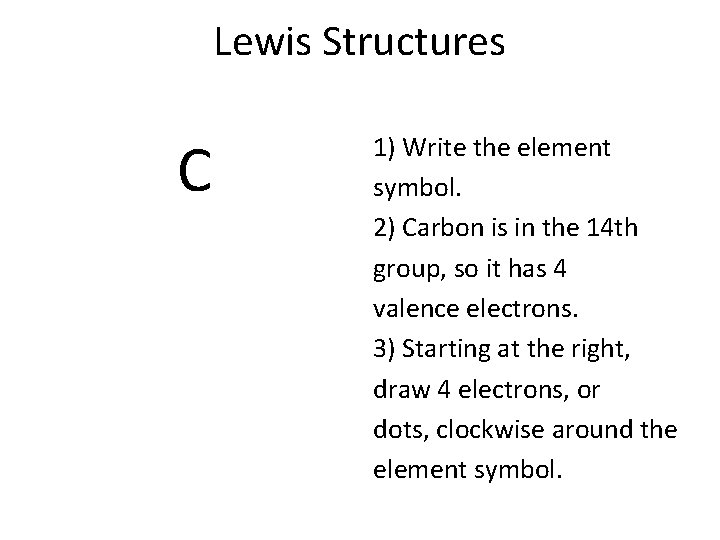
Lewis Structures C 1) Write the element symbol. 2) Carbon is in the 14 th group, so it has 4 valence electrons. 3) Starting at the right, draw 4 electrons, or dots, clockwise around the element symbol.
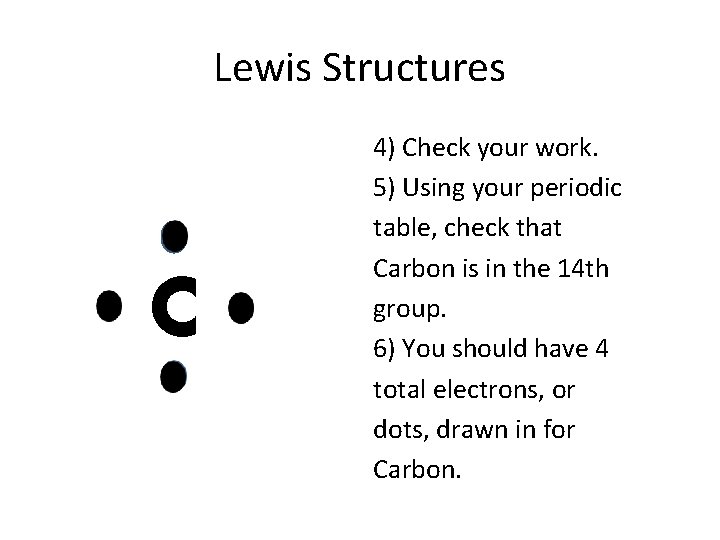
Lewis Structures C 4) Check your work. 5) Using your periodic table, check that Carbon is in the 14 th group. 6) You should have 4 total electrons, or dots, drawn in for Carbon.

Lewis Dot • Use your Bohr Diagram and Just diagram the valence shell (shell furthest away from the nucleus). LET’S PRACTICE
• Let’s do Find it on periodic table… how many shells? How many VALENCE electrons? BOHR DIAGRAM Atomic # (which means #Protons) Electrons (matches the # of Protons) K -First Shell L -Second Shell M -Third Shell Lewis Dot Structure
1. Find it on periodic table… how many shells? Argon How many VALENCE electrons? Atomic # = # of Electrons = K -First Shell = L -Second Shell = M -Third Shell = BOHR DIAGRAM
- Slides: 27