How to download Accelrys DS visualizer http accelrys








- Slides: 22

How to download Accelrys DS visualizer http: //accelrys. com/resourcecenter/downloads/freeware/index. html 2. Click on Learn more and request a free copy of Discovery Studio Visualizer 3. Complete the form to receive your free version of DS Visualizer. 4. You will receive an email from the company containing the link to download the software. 1.

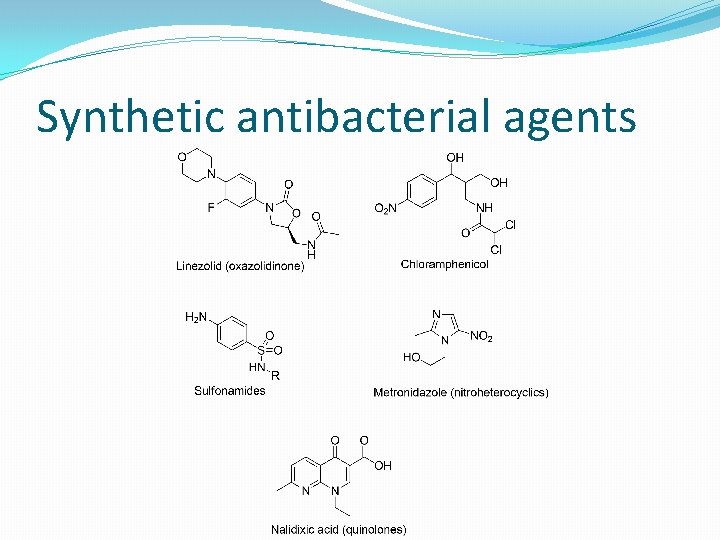
Synthetic antibacterial agents

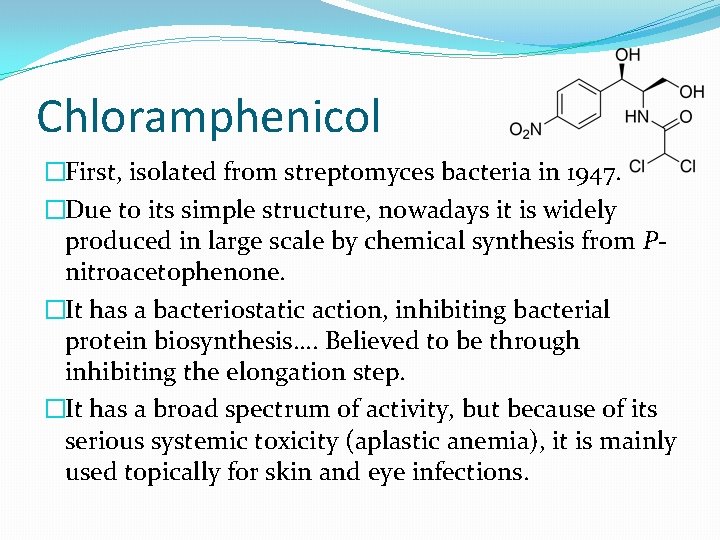
Chloramphenicol �First, isolated from streptomyces bacteria in 1947. �Due to its simple structure, nowadays it is widely produced in large scale by chemical synthesis from Pnitroacetophenone. �It has a bacteriostatic action, inhibiting bacterial protein biosynthesis…. Believed to be through inhibiting the elongation step. �It has a broad spectrum of activity, but because of its serious systemic toxicity (aplastic anemia), it is mainly used topically for skin and eye infections.

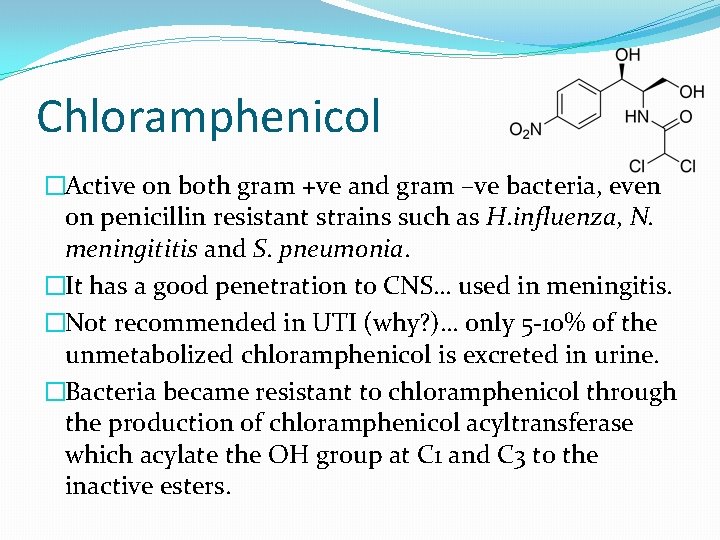
Chloramphenicol �Active on both gram +ve and gram –ve bacteria, even on penicillin resistant strains such as H. influenza, N. meningititis and S. pneumonia. �It has a good penetration to CNS… used in meningitis. �Not recommended in UTI (why? )… only 5 -10% of the unmetabolized chloramphenicol is excreted in urine. �Bacteria became resistant to chloramphenicol through the production of chloramphenicol acyltransferase which acylate the OH group at C 1 and C 3 to the inactive esters.

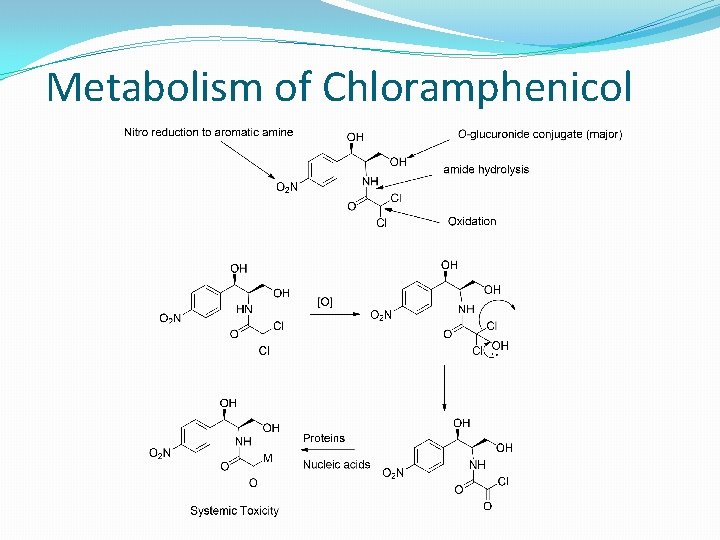
Metabolism of Chloramphenicol

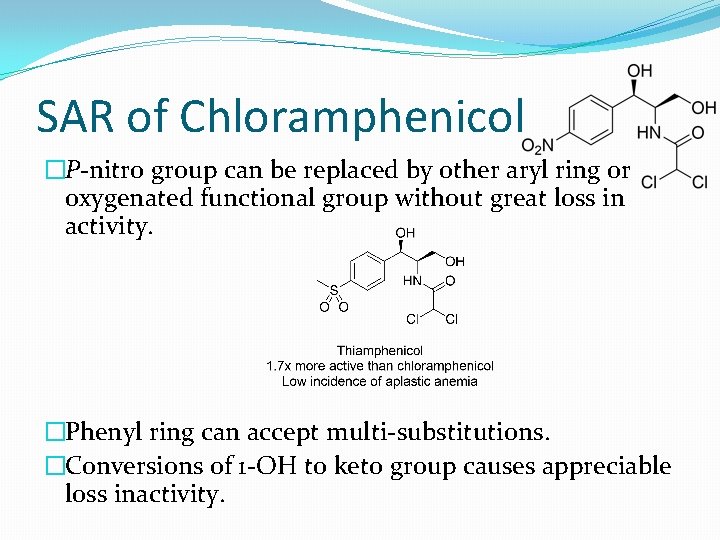
SAR of Chloramphenicol �P-nitro group can be replaced by other aryl ring or oxygenated functional group without great loss in activity. �Phenyl ring can accept multi-substitutions. �Conversions of 1 -OH to keto group causes appreciable loss inactivity.

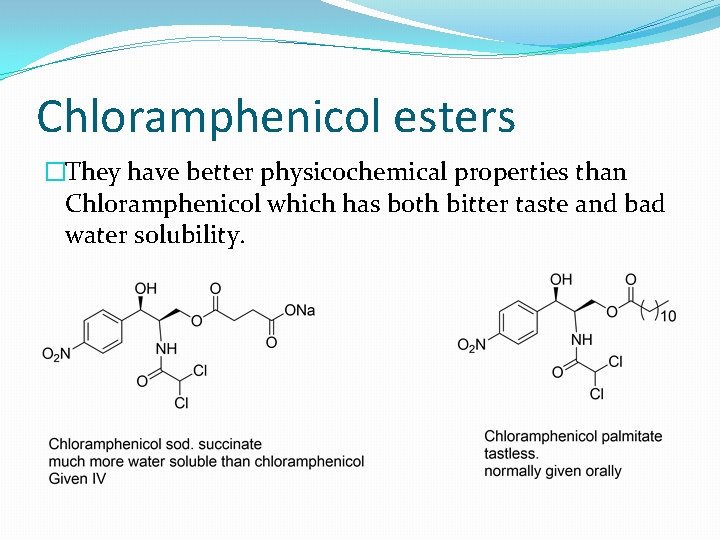
Chloramphenicol esters �They have better physicochemical properties than Chloramphenicol which has both bitter taste and bad water solubility.

Quinolones antibacterial agents �Nalidixic acid is the lead compound for this group. �According to the heterocyclic core can be divided into: �Naphthyridines: nalidixic acid and enoxacin. �Quinolines: norfloxacin, ciprofloxacin, lemofloxacin. �Cinnolines: Cinoxacin

Quinolones antibacterial agents �Spectrum of activity: �Highly active against urinary tract pathogens such as E. coli, Klebsiella, Citrobacter, proteus as well as salmonella and shigella. �Most except fluoroquinolones are not active on P. aeruginosa and H. influenza. �Inactive on anaerobic and gram +ve bacteria. �Pharmacokinetics: �They have good oral bioavailability. �They reach urine in enough concentration to be effective in UTI (>40%).

Quinolones antibacterial agents �Mechanism of action: Inhibit DNA synthesis by inhibiting DNA gyrase (topoisomerase-II) which is important for DNA supercoiling. �Transport into bacterial cell: mainly through the porin channels of the gram –ve bacteria. �Mechanisms of bacterial resistance: �Mutation in porin channels. �Energy dependant efflux mechanism.

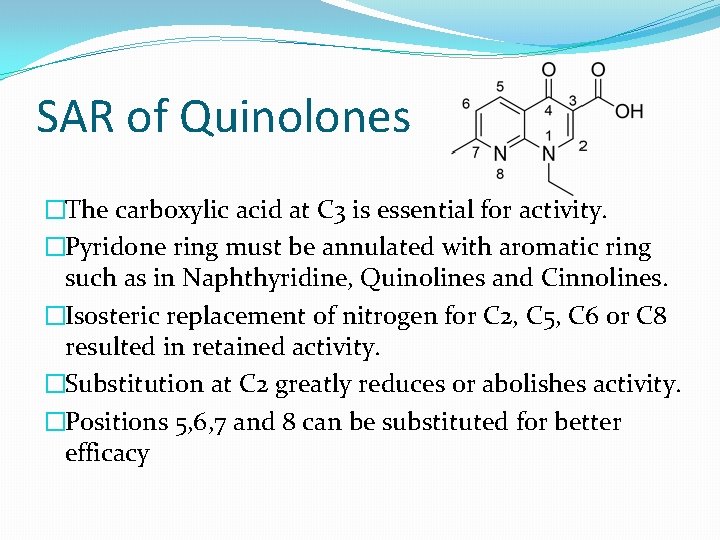
SAR of Quinolones �The carboxylic acid at C 3 is essential for activity. �Pyridone ring must be annulated with aromatic ring such as in Naphthyridine, Quinolines and Cinnolines. �Isosteric replacement of nitrogen for C 2, C 5, C 6 or C 8 resulted in retained activity. �Substitution at C 2 greatly reduces or abolishes activity. �Positions 5, 6, 7 and 8 can be substituted for better efficacy

SAR of Quinolones �Positions 5, 6, 7 and 8 can be substituted for better efficacy: �Piperazine ring and 3 -aminopyrrolidine at C 7 enhances activity, mainly against P. aeruginosa. �Fluorine atom at C 6 also improved activity (Fluoroquinolones). �Alkyl substitution on C 1 improves activity (but small alkyl or aryl group). �Ring condensation at 1 -8, 5 -6, 6 -7 and 7 -8 also lead to better activity.

Fluoroquinolones �They are 6 -fluoro-7 -piperazinoquinolones derivatives. �They exhibit extended spectrum of activity that covers most of gram +ve and gram –ve bacteria especially P. aeruginosa. �Members: �Ciprofloxacin. �Norfloxacin. �Ofloxacin. �Pefloxacin. �Lomefloxacin. �Enofloxacin. �Levofloxacin.

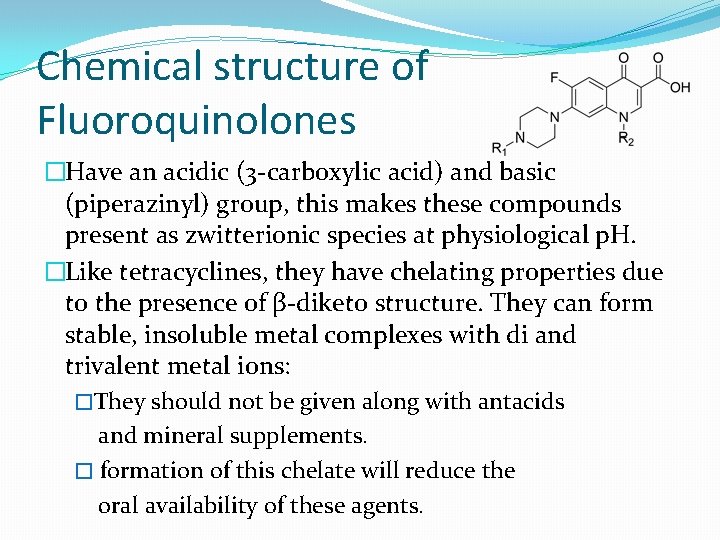
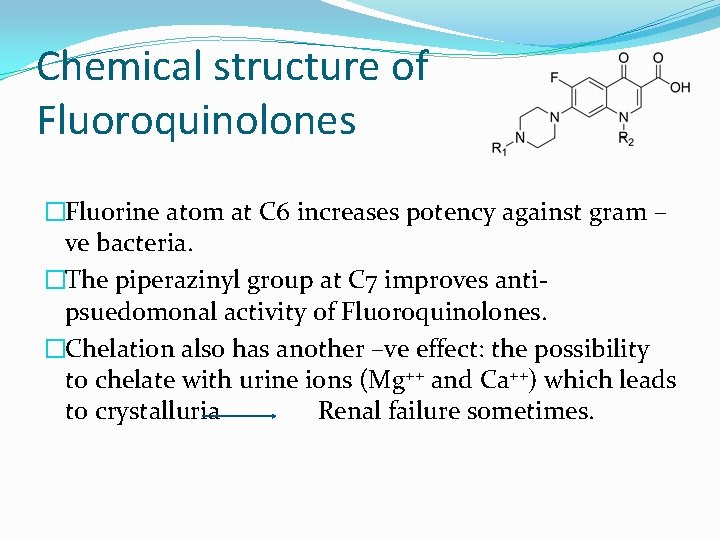
Chemical structure of Fluoroquinolones �Have an acidic (3 -carboxylic acid) and basic (piperazinyl) group, this makes these compounds present as zwitterionic species at physiological p. H. �Like tetracyclines, they have chelating properties due to the presence of β-diketo structure. They can form stable, insoluble metal complexes with di and trivalent metal ions: �They should not be given along with antacids and mineral supplements. � formation of this chelate will reduce the oral availability of these agents.


Chemical structure of Fluoroquinolones �Fluorine atom at C 6 increases potency against gram – ve bacteria. �The piperazinyl group at C 7 improves antipsuedomonal activity of Fluoroquinolones. �Chelation also has another –ve effect: the possibility to chelate with urine ions (Mg++ and Ca++) which leads to crystalluria Renal failure sometimes.

Enoxacin �Well absorbed following oral administration (90%). �Well distributed through the body… reaches kidney, prostate and cervix. �Used mainly in prostatitis. Ciprofloxacin � 40 -50% excreted unchanged in urine. �Highly distributed to all body fluids including CS fluid. �Highly potent against gram –ve especially P. aeruginosa (why? ). �Used in gastroenteritis, skin, soft tissues (bone and joints) infections and UTI.

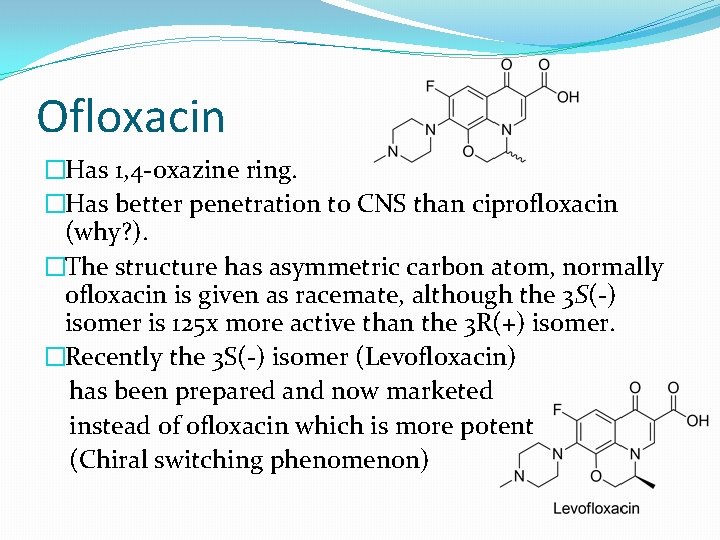
Ofloxacin �Has 1, 4 -oxazine ring. �Has better penetration to CNS than ciprofloxacin (why? ). �The structure has asymmetric carbon atom, normally ofloxacin is given as racemate, although the 3 S(-) isomer is 125 x more active than the 3 R(+) isomer. �Recently the 3 S(-) isomer (Levofloxacin) has been prepared and now marketed instead of ofloxacin which is more potent (Chiral switching phenomenon)

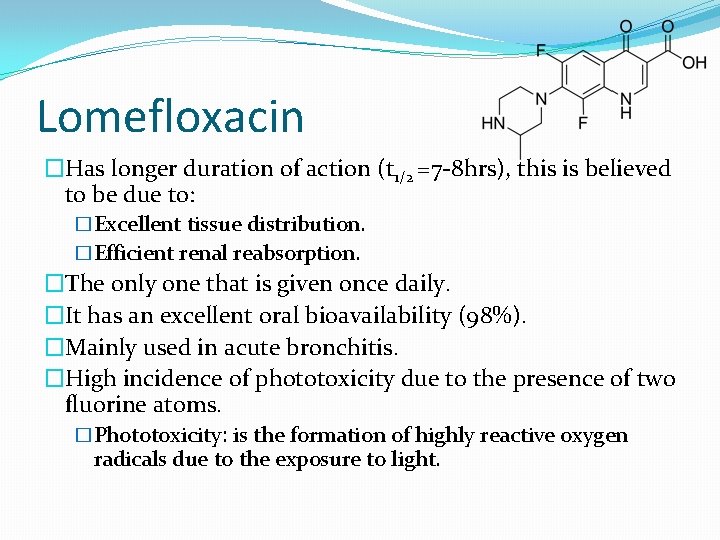
Lomefloxacin �Has longer duration of action (t 1/2 =7 -8 hrs), this is believed to be due to: �Excellent tissue distribution. �Efficient renal reabsorption. �The only one that is given once daily. �It has an excellent oral bioavailability (98%). �Mainly used in acute bronchitis. �High incidence of phototoxicity due to the presence of two fluorine atoms. �Phototoxicity: is the formation of highly reactive oxygen radicals due to the exposure to light.

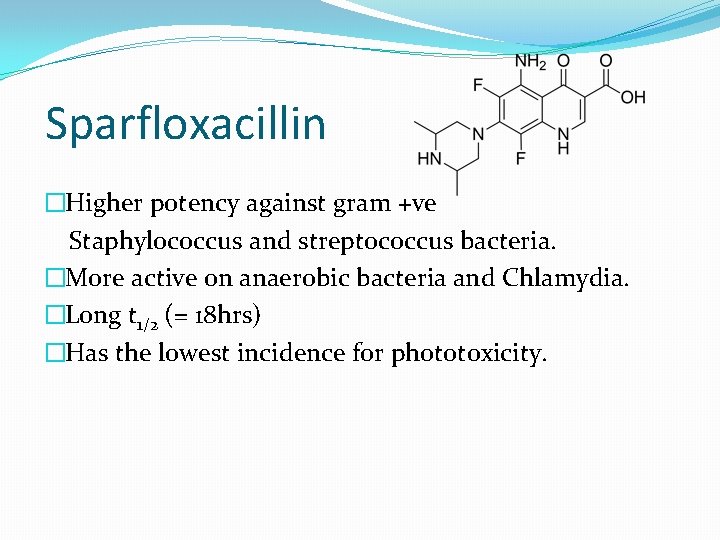
Sparfloxacillin �Higher potency against gram +ve Staphylococcus and streptococcus bacteria. �More active on anaerobic bacteria and Chlamydia. �Long t 1/2 (= 18 hrs) �Has the lowest incidence for phototoxicity.

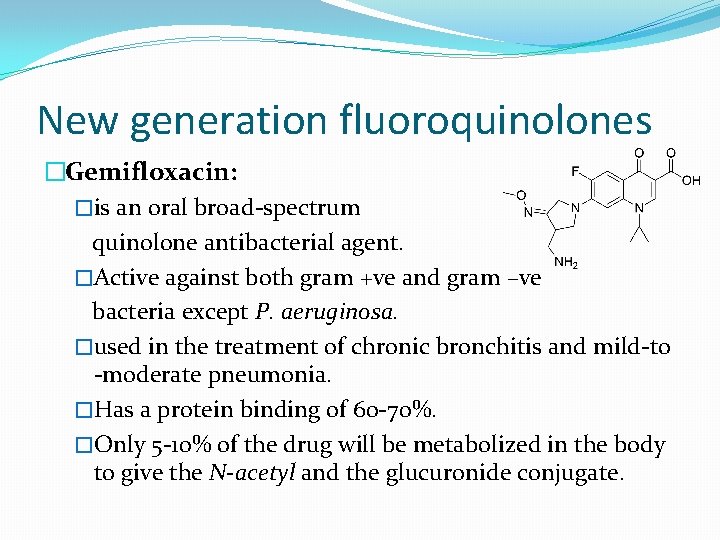
New generation fluoroquinolones �Gemifloxacin: �is an oral broad-spectrum quinolone antibacterial agent. �Active against both gram +ve and gram –ve bacteria except P. aeruginosa. �used in the treatment of chronic bronchitis and mild-to -moderate pneumonia. �Has a protein binding of 60 -70%. �Only 5 -10% of the drug will be metabolized in the body to give the N-acetyl and the glucuronide conjugate.

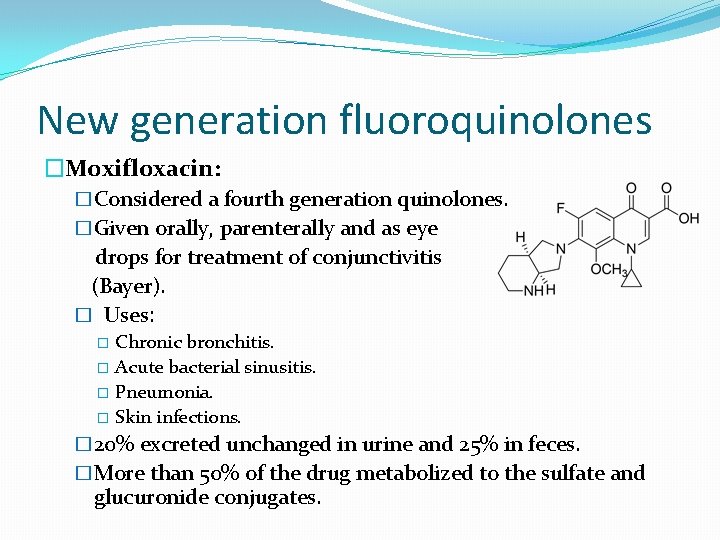
New generation fluoroquinolones �Moxifloxacin: �Considered a fourth generation quinolones. �Given orally, parenterally and as eye drops for treatment of conjunctivitis (Bayer). � Uses: Chronic bronchitis. � Acute bacterial sinusitis. � Pneumonia. � Skin infections. � � 20% excreted unchanged in urine and 25% in feces. �More than 50% of the drug metabolized to the sulfate and glucuronide conjugates.