How to detect How can one tell if












- Slides: 19

How to detect? How can one tell if a solution is present by looking? (1 min to brainstorm) Ideas?

Solutions to test Tap water from our sink Distilled water Vitamin water “hard” water from the Feist family well Mineral water 0. 01 M salt, 0. 5 M salt, 1. 0 M salt

Methods to determine Evaporation Conductivity Sedimentation/coagulation Filter

Detecting solutions 1

Detecting solutions 2 Faster than the water spot test is the conductivity tester. Dip the wire end into the distilled water and dry. Dip the wire end into the solution being tested. Compare the sound of the tester when the concentration of solution changes.

Solutions 3 Remember how to fold filter paper? Filter 15 m. L of the solutions tested in the previous activity. (each group will do different solutions) Take the solution that comes through the filter and repeat the slide test and conductivity tests.

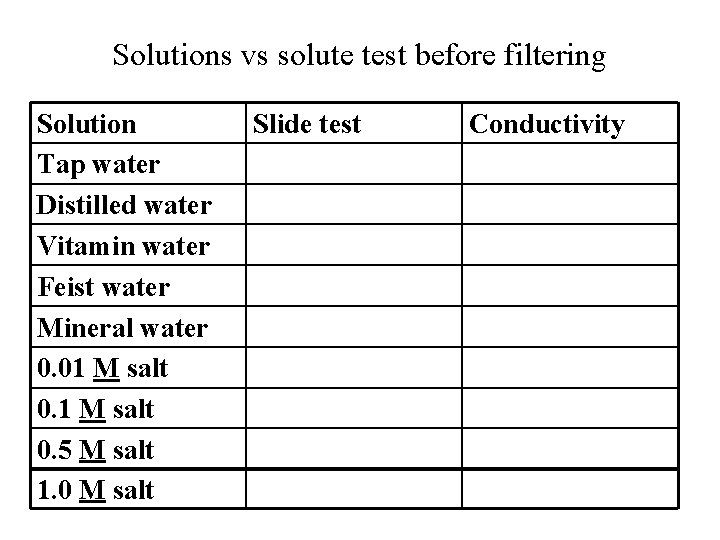
Solutions vs solute test before filtering Solution Tap water Distilled water Vitamin water Feist water Mineral water 0. 01 M salt 0. 5 M salt 1. 0 M salt Slide test Conductivity

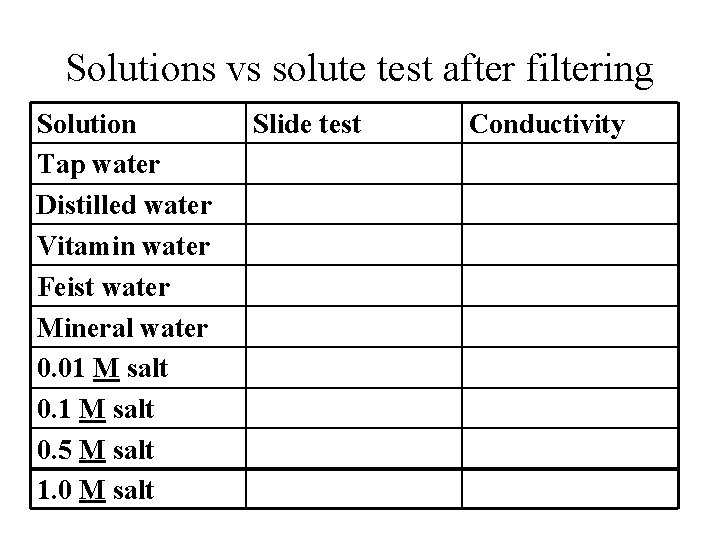
Solutions vs solute test after filtering Solution Tap water Distilled water Vitamin water Feist water Mineral water 0. 01 M salt 0. 5 M salt 1. 0 M salt Slide test Conductivity

Purifying Methods Filtering Simple ion exchange Sedimentation Chlorination Distillation

Filtering & Purifying Filter 2 of the water samples and decide if filtering was sufficient to clean the water. What tests can you do to determine if the filtered water is clean or purified?

Purifying 1 Divide the filtered water into 2 samples; see below. Look at the next 2 slides and follow the instructions. Answer the questions too. Filtered hot water 1 Filtered hot water 2 Filtered blue water 1 Filtered blue water 2

Chlorination

Distillation

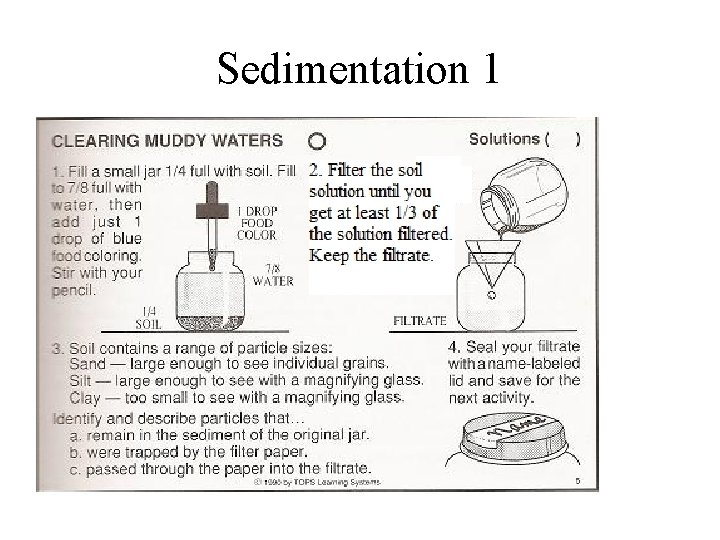
Sedimentation 1

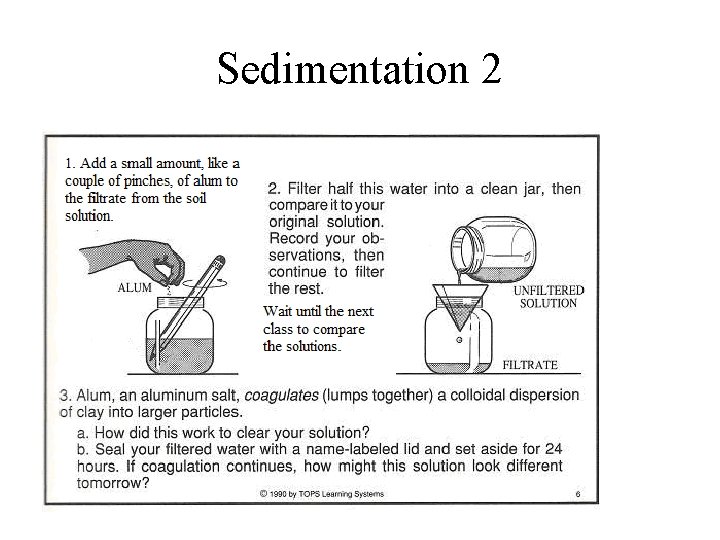
Sedimentation 2

Sedimentation 3 Does sedimentation clean the soil and water solution? How can you tell? What tests have you done to be certain the water is clean or pure?

How to separate solutions Separating Solutions

Separating by size Chromatography Chroma- means: -graph means: Sharpies® are considered “permanent”. Why?

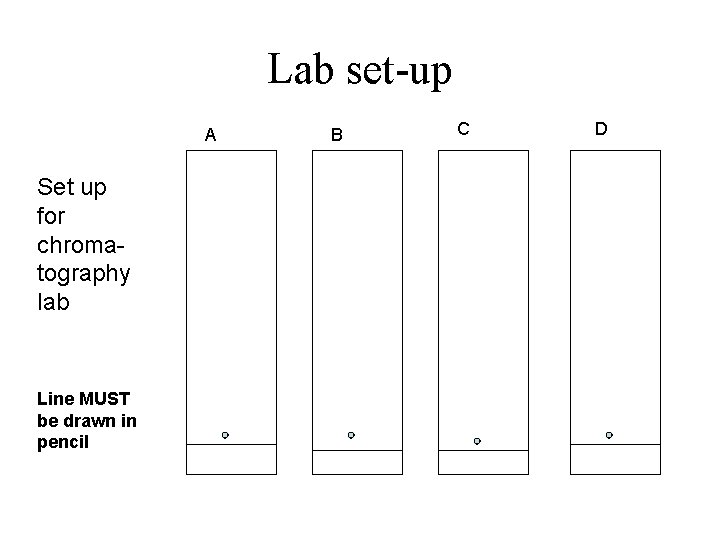
Lab set-up A Set up for chromatography lab Line MUST be drawn in pencil B C D