HOW TO CONDUCT EFFECTIVE COMPLIANCE INVESTIGATIONS TEXAS COUNCIL


































- Slides: 53

HOW TO CONDUCT EFFECTIVE COMPLIANCE INVESTIGATIONS TEXAS COUNCIL OF COMMUNITY CENTERS ANNUAL CONFERENCE JUNE 20, 2018 PANEL PRESENTATION

INTRODUCTIONS PANEL PRESENTERS RENE NAVARRRO Chief Compliance Officer Emergence Health Network RIK LINDAHL Director, Compliance, Planning and Quality Assurance Lifepath Systems DONNA MOORE Director of Quality Management Burke FACILITATOR RATANA DELUCA Chief Compliance Officer Metrocare Services SAROEUN SVAY Compliance Manager Metrocare Services

DISCLAIMER Any statements, recommendations, and opinions made by the Presenters in and during this presentation are our own, and do not belong to our respective Centers or otherwise are not reflective of each of our Centers’ stance, position, or reputation. This presentation is meant to serve as education and guidance tool, and not meant to be construed as professional or legal advice.

DISCUSSION TOPICS • Nature and Scope of Compliance Investigations • Professional Guidance on Compliance Investigations • Application of Professional Guidance within Community Centers • Recommendations of Effective Practices for Community Centers

LEARNING OBJECTIVES • Become familiar with and learn about the types of compliance investigation practices that have been recommended or deemed highly effective across all organizations; • Analyze how the recommended compliance investigation practices have been applied and worked within the different types of community centers settings; and • Determine what compliance investigation practices will be suitable for your center.

COMPLIANCE INVESTIGATION OVERVIEW What is a Compliance Investigation? Review of a matter that is a potential or actual violation of a policy, contract, regulation, or law. Internal agency policies Government regulatory policies Private contracts Programmatic contracts with government Local, State or federal regulation

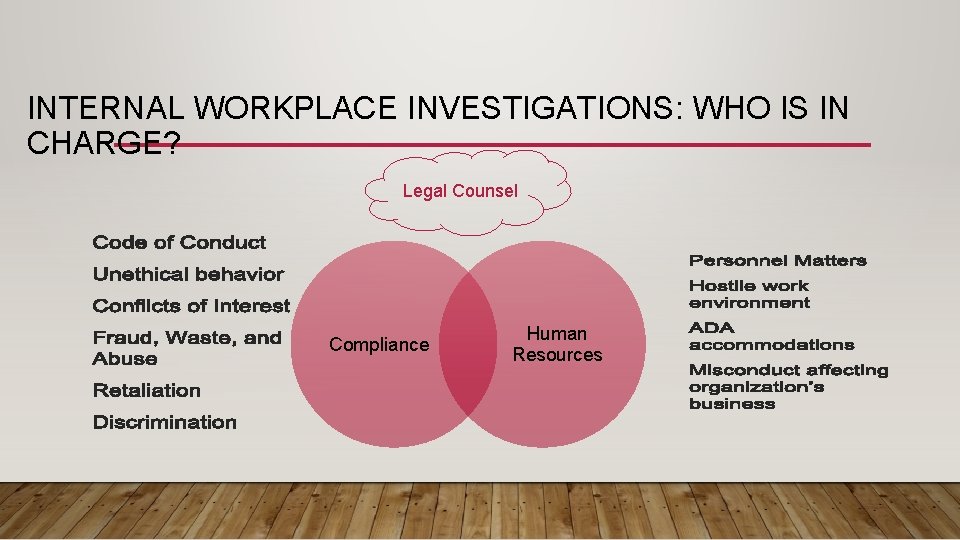
INTERNAL WORKPLACE INVESTIGATIONS: WHO IS IN CHARGE? Legal Counsel Compliance Human Resources

COMPLIANCE INVESTIGATION AREAS Code of Conduct Fraud, Waste, Abuse Conflict of Interest Unprofessional boundaries Irregular Documentation Ownership Interest Unethical business practices Fraudulent, falsifying documents Improper Referrals Discrimination Misuse of public funds/resources Accepting gifts or items of value This presentation will address only investigation processes and techniques internal to its Center

NECESSITY OF COMPLIANCE INVESTIGATION Prevent/Mitigate Immediate Harm • Physical • Financial • Reputational Good Faith Effort Demonstration • Proactiveness • Corrective action taken timely Favorable Considerations by Government • Penalty reductions • Less harsh sanctions/adverse actions Regulatory agencies look to see whethere is a compliance department or designee in place to conduct workplace investigations and whether compliance investigations were done.

COMPLIANCE INVESTIGATIONS BEST PRACTICES PROFESSIONAL RESOURCES U. S. Office of Inspector General U. S. Department of Justice Health Care Compliance Association Texas Council Risk Management Fund American Health Lawyers Association

PROFESSIONAL GUIDANCE BEST PRACTICES SUMMARY Establish written investigation plan, policies, procedures, and protocols Investigation practices are consistent Mechanism in place for reporting compliance matters Methodology for measuring effectiveness of investigation process Investigating staff are adequately trained, and provided with resources, support, and authority to work independently Protections in place for investigators, witnesses, and others taking part in investigation (directly or indirectly) Investigation documentation is completed and maintained based on record retention policies

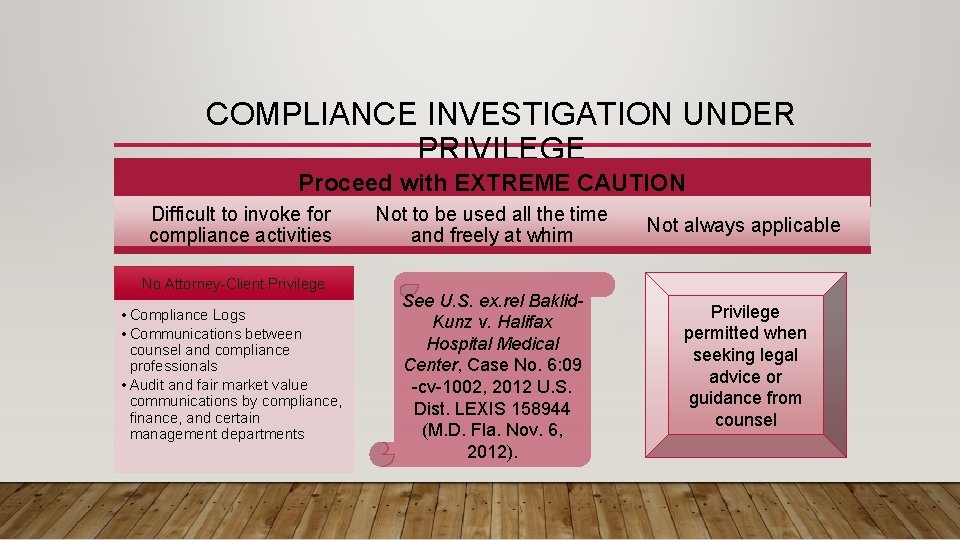
COMPLIANCE INVESTIGATION UNDER PRIVILEGE Proceed with EXTREME CAUTION Difficult to invoke for compliance activities No Attorney-Client Privilege • Compliance Logs • Communications between counsel and compliance professionals • Audit and fair market value communications by compliance, finance, and certain management departments Not to be used all the time and freely at whim See U. S. ex. rel Baklid. Kunz v. Halifax Hospital Medical Center, Case No. 6: 09 -cv-1002, 2012 U. S. Dist. LEXIS 158944 (M. D. Fla. Nov. 6, 2012). Not always applicable Privilege permitted when seeking legal advice or guidance from counsel

WHY NOT HAVE COUNSEL CONDUCT INVESTIGATION? Costs Knowledge Interaction No guarantee of privilege Compliance professional must be the intermediary between legal and the organization to conduct investigation and know when to reach out to counsel and management

COMMUNITY CENTER COMPLIANCE INVESTIGATION PRACTICES Community Center Compliance Investigation Practices • Investigation Techniques/Practices Overview • Effectiveness of Practices • Ineffective Practices

Compliance Investigation Practices Presented by Donna Moore, Director of Quality Management

Overview • Compliance Officer • Compliance Committee • Types of issues investigated: • False billing • False documentation • Anti-Kickback Statute • Misuse of Resources • Conflict of interests • Other Code of Conduct violations

Process There always two issues to be addressed: • Is there a violation? • What needs to be done?

Investigative Process • Intake • Action Plan • Fact Finding • Interview of subject • Analysis • Report • Corrective Actions

Effective Processes • Data, documents, and reports • Knowledge of service provision • Contact with individuals served • Constant education on corporate compliance topics • Be visible and accessible

Ineffective Processes • Using the wrong people to help • Relying on the medical record • Failing to attend to red flags • Drawing conclusions too quickly

Lessons Learned • Have friends outside of work • Be a resource • You can’t educate staff too much • You can’t be over-qualified • Build credibility and support • Educate your leaders

Life. Path Systems Building stronger communities, person by person. Compliance Investigation Practices Rik Lindahl Director, Compliance, Planning and Quality Assurance

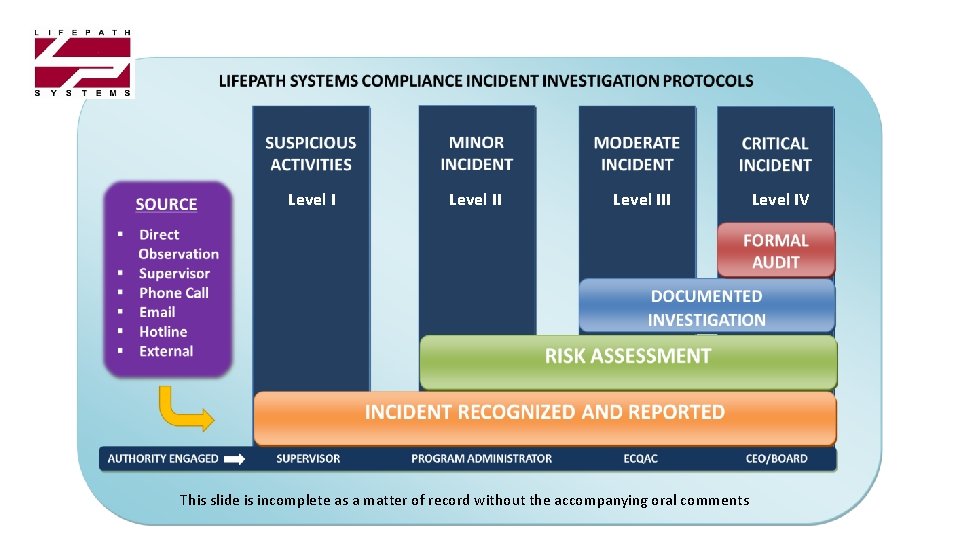
Level III This slide is incomplete as a matter of record without the accompanying oral comments Level IV

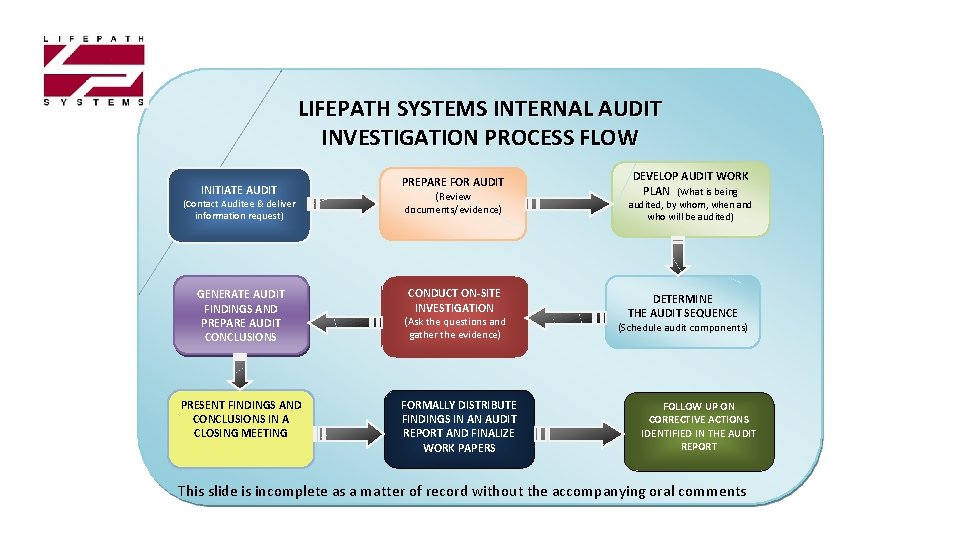
LIFEPATH SYSTEMS INTERNAL AUDIT INVESTIGATION PROCESS FLOW INITIATE AUDIT (Contact Auditee & deliver information request) GENERATE AUDIT FINDINGS AND PREPARE AUDIT CONCLUSIONS PRESENT FINDINGS AND CONCLUSIONS IN A CLOSING MEETING PREPARE FOR AUDIT (Review documents/evidence) CONDUCT ON-SITE INVESTIGATION (Ask the questions and gather the evidence) FORMALLY DISTRIBUTE FINDINGS IN AN AUDIT REPORT AND FINALIZE WORK PAPERS DEVELOP AUDIT WORK PLAN (What is being audited, by whom, when and who will be audited) DETERMINE THE AUDIT SEQUENCE (Schedule audit components) FOLLOW UP ON CORRECTIVE ACTIONS IDENTIFIED IN THE AUDIT REPORT This slide is incomplete as a matter of record without the accompanying oral comments

METROCARE SERVICES COMPLIANCE INVESTIGATION PRACTICES Saroeun Svay Compliance Manager Metrocare Services

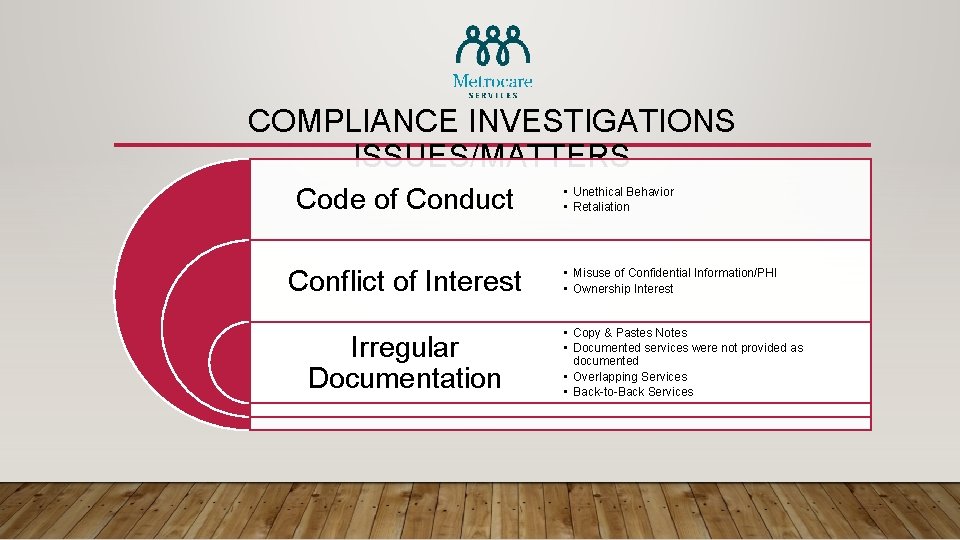
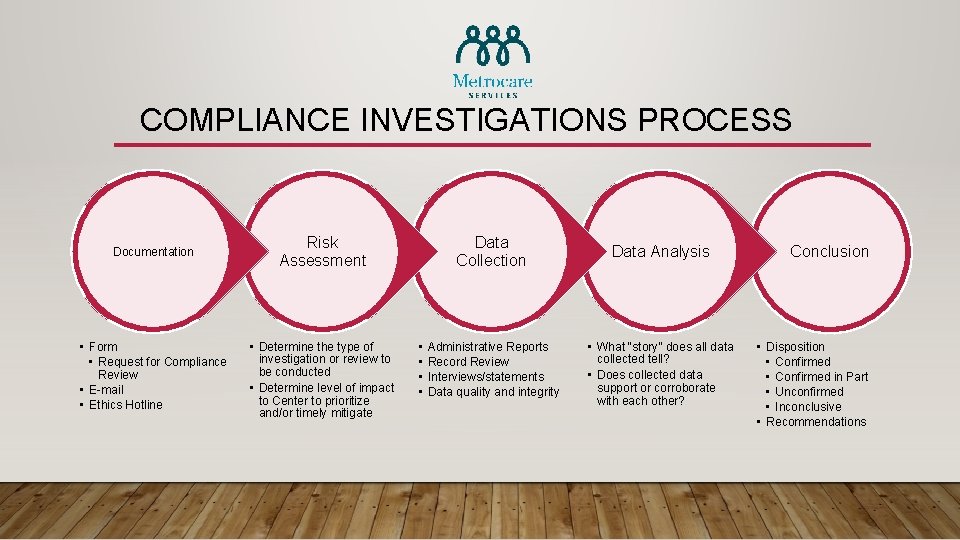
COMPLIANCE INVESTIGATION OVERVIEW Issues/Matters • Code of Conduct • Conflict of Interest • Irregular Documentation Reporting • • In-Person Telephone E-Mail Ethics Hotline Process • • Risk Assessment Data Collection Data Analysis Conclusion

COMPLIANCE INVESTIGATIONS ISSUES/MATTERS Code of Conduct Conflict of Interest Irregular Documentation • Unethical Behavior • Retaliation • Misuse of Confidential Information/PHI • Ownership Interest • Copy & Pastes Notes • Documented services were not provided as documented • Overlapping Services • Back-to-Back Services

COMPLIANCE INVESTIGATIONS - REPORTING In-Person Telephone E-Mail Ethics Hotline

COMPLIANCE INVESTIGATIONS PROCESS Documentation Risk Assessment • Form • Request for Compliance Review • E-mail • Ethics Hotline • Determine the type of investigation or review to be conducted • Determine level of impact to Center to prioritize and/or timely mitigate • • Data Collection Data Analysis Administrative Reports Record Review Interviews/statements Data quality and integrity • What “story” does all data collected tell? • Does collected data support or corroborate with each other? Conclusion • Disposition • Confirmed in Part • Unconfirmed • Inconclusive • Recommendations

EFFECTIVE INVESTIGATION PRACTICES Documentation Timing Focus Collaboration

INEFFECTIVE INVESTIGATION PRACTICES • Various Reports due to lack of submitted data • Lack of cooperation • Non-responsiveness from witnesses/interviewees • Timeliness review of collected data

LESSONS LEARNED • Focus on the issue that has been reported • Conduct interviews/phone calls as soon as possible while information is still fresh • Review collected data as soon as possible • Complete data analysis timely

Rene Navarro, MPA, CHP Chief Compliance Officer (915)887 -3410 ext. 18819 rnavarro@ehnelpaso. org Compliance Investigations Practices

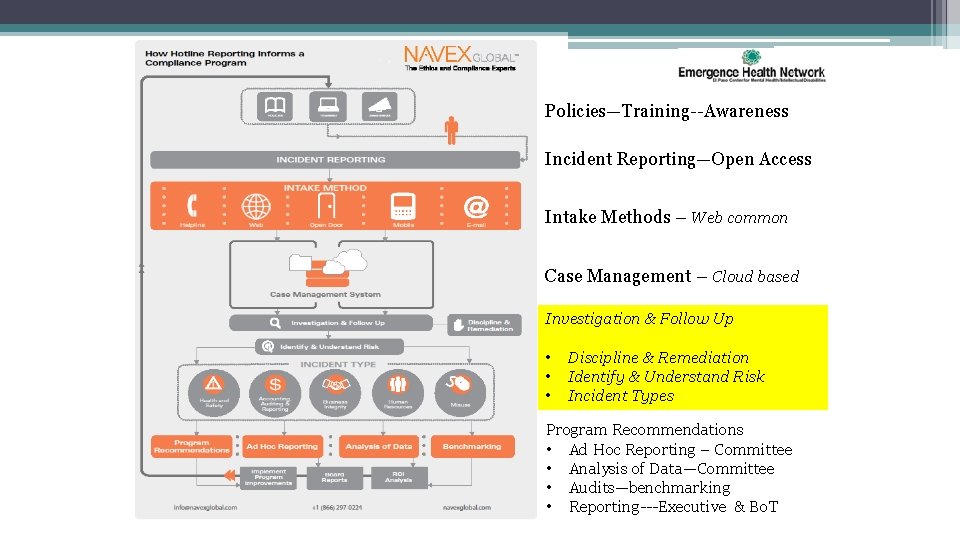
Policies—Training--Awareness Incident Reporting—Open Access Intake Methods – Web common Case Management – Cloud based Investigation & Follow Up • • • Discipline & Remediation Identify & Understand Risk Incident Types Program Recommendations • Ad Hoc Reporting – Committee • Analysis of Data—Committee • Audits—benchmarking • Reporting---Executive & Bo. T

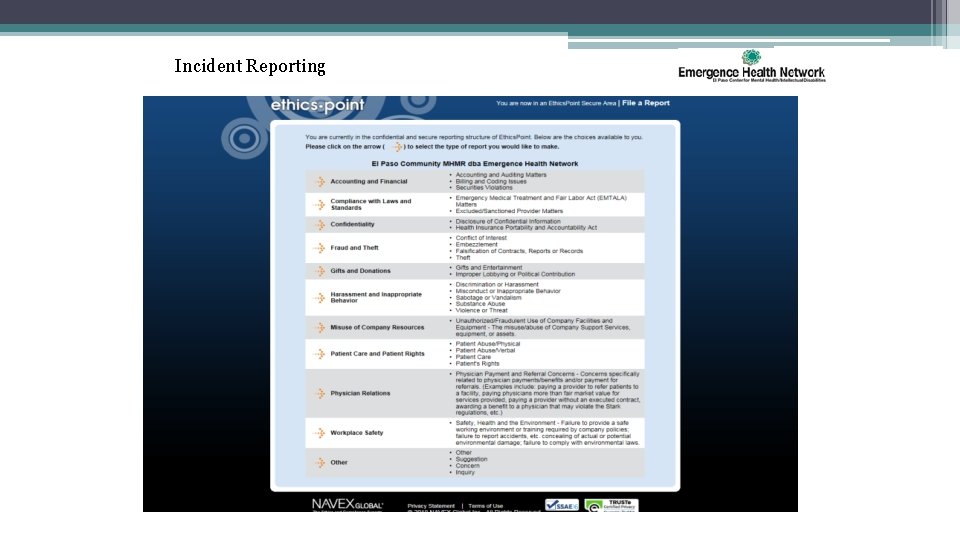
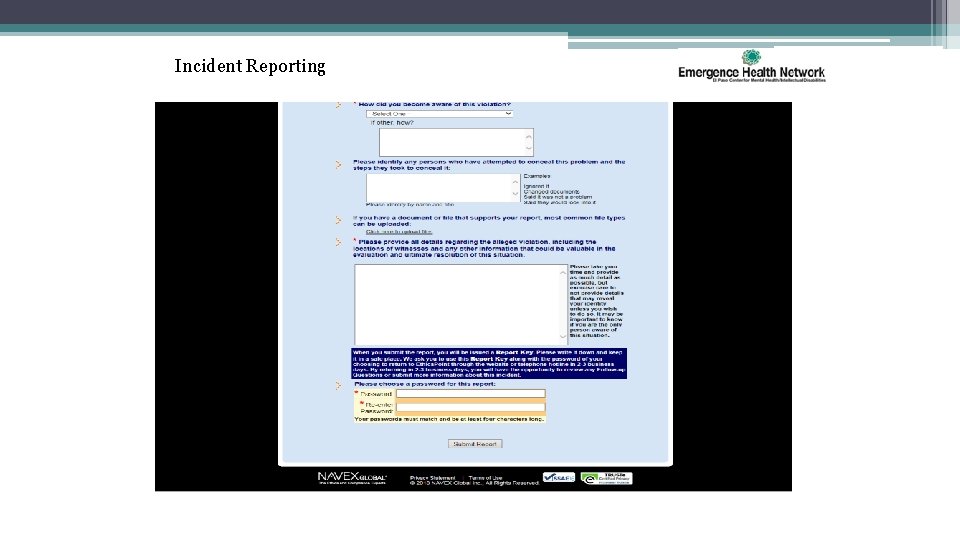
Incident Reporting

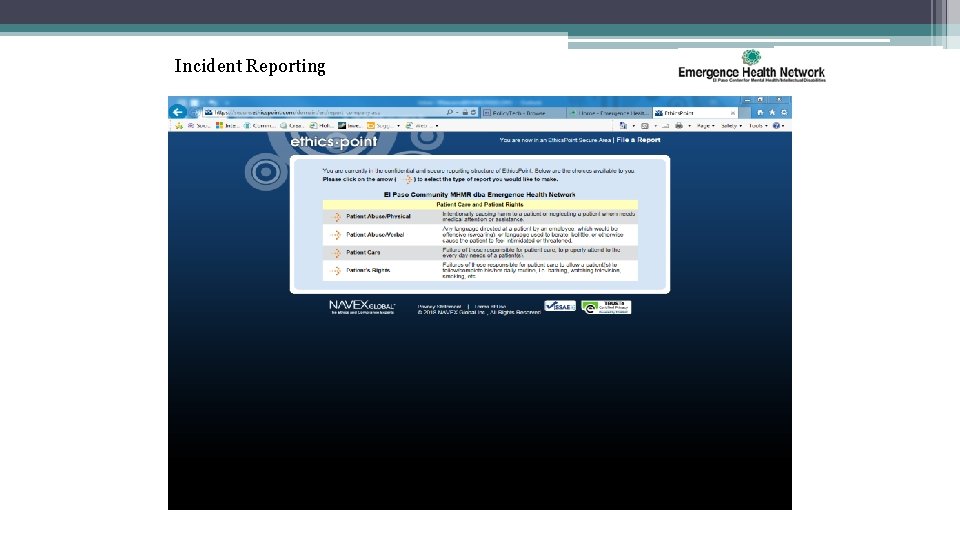
Incident Reporting

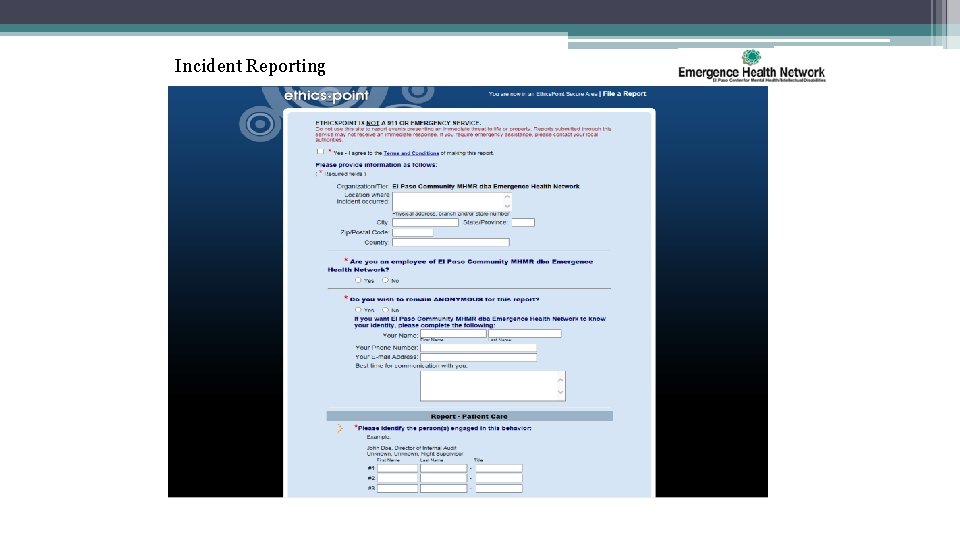
Incident Reporting

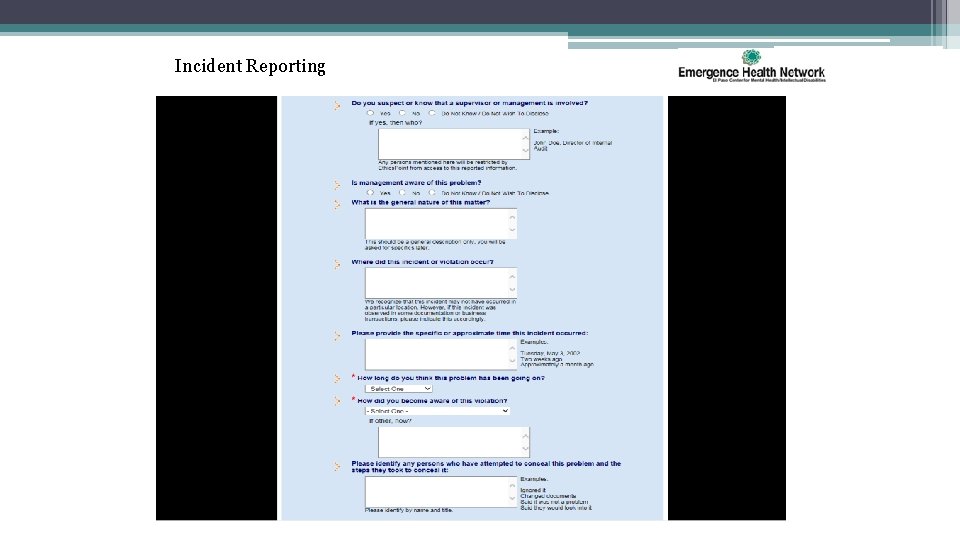
Incident Reporting

Incident Reporting

Incident Reporting

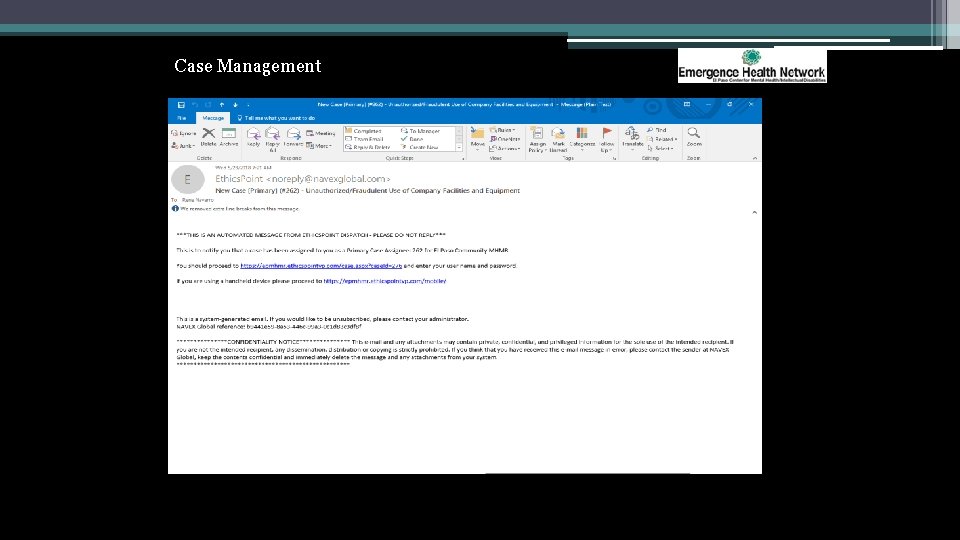
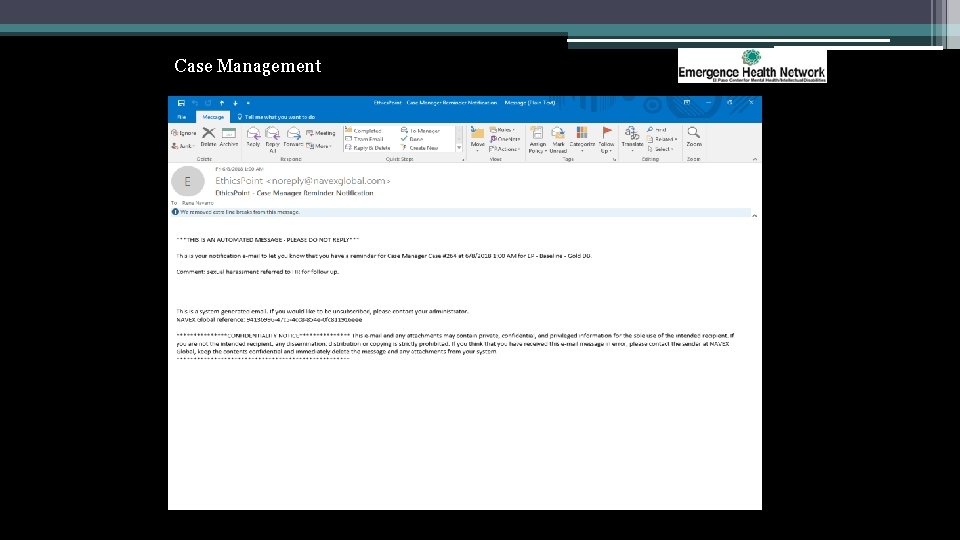
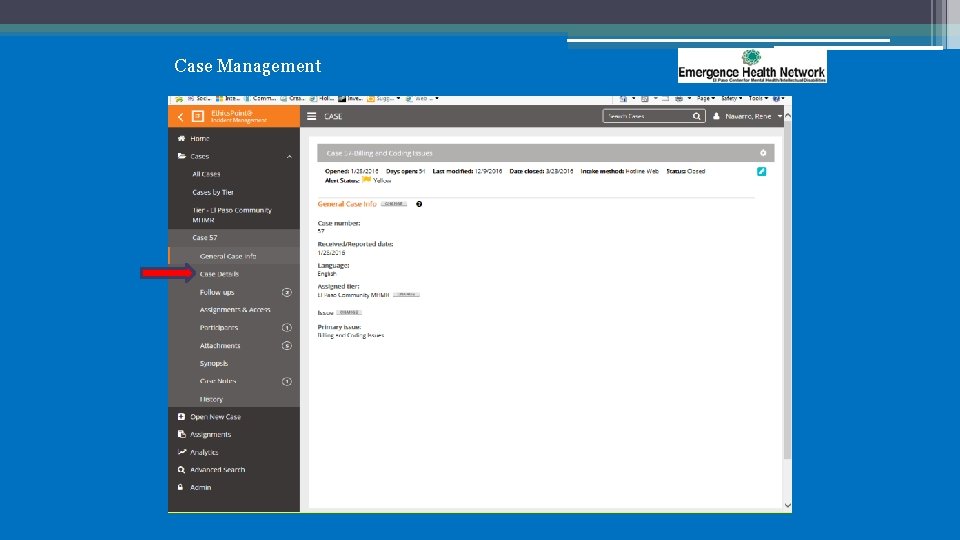
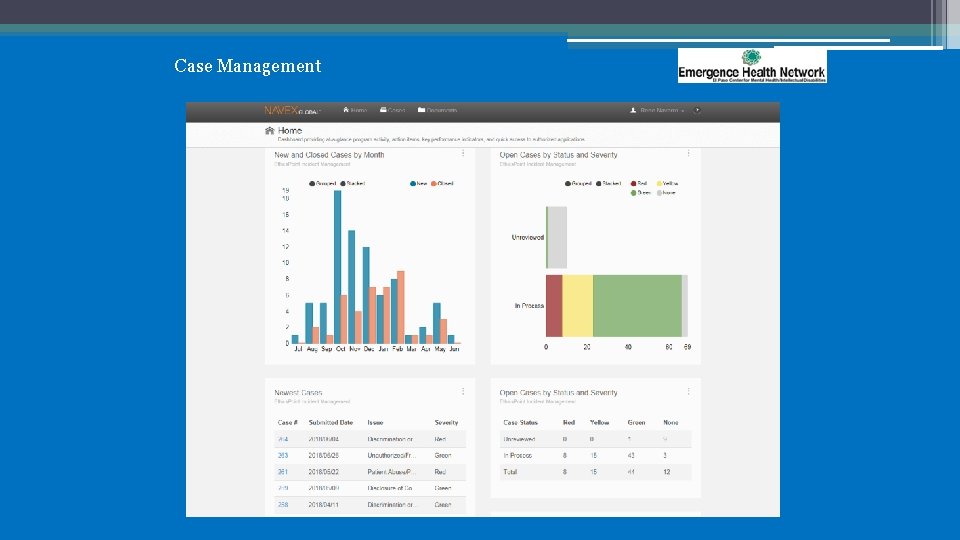
Case Management

Case Management

Case Management

Case Management

Case Management

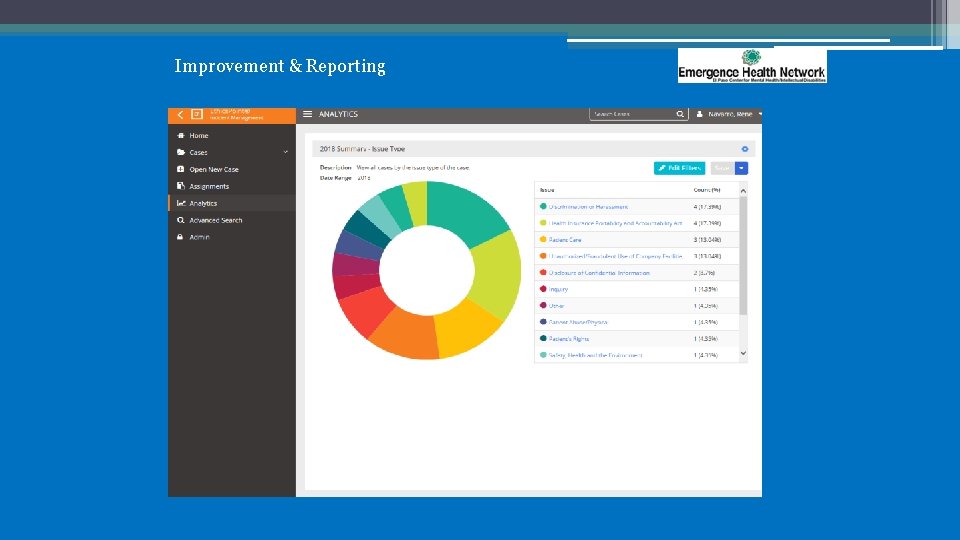
Improvement & Reporting

COMMUNITY CENTER COMMON INVESTIGATION PRACTICES Designated trained and experienced staff Conducting risk assessments of matters reported Have proper reporting channels in place Investigation plan and protocols Interdepartmental collaboration during investigation Emphasis on consistency on investigation process Collection of data/information from various resources for validation, integrity, and totality Method for tracking and evaluating effectiveness of compliance investigations Management reporting of compliance investigations

RECOMMENDED INVESTIGATION PRACTICES FOR COMMUNITY CENTERS Effective Compliance Investigation Practice Recommendations Designated staff person to conduct compliance investigations Provide ongoing, appropriate training, resources, and support to ensure effective investigations done Develop an investigation plan and investigation protocols that demonstrate an independent, consistent, and objective Evaluate process for effectiveness at least annually and flexible to meet any exigent or exceptional circumstances Ensure data collected and communications are maintained, properly stored, documented, and kept confidential Ensure integrity, validation, and quality of data and information collected Collection of data and information from multiple sources that would corroborate with each other Know when to seek advice and guidance from counsel or other professionals

COMPLIANCE INVESTIGATION PRACTICES TO AVOID Compliance Investigation Practices to Avoid Rushing an investigation Not utilizing narrative, open-ended questions Inconsistent questioning, treatment, and information gathering of witnesses Undocumented investigation plans and processes Changing processes too frequently Reliance on only one source of information Gathering information indirectly or deemed as opinions Not enforcing confidentiality or anti-retaliation policies Not consulting with counsel or appropriate individuals or resources for guidance

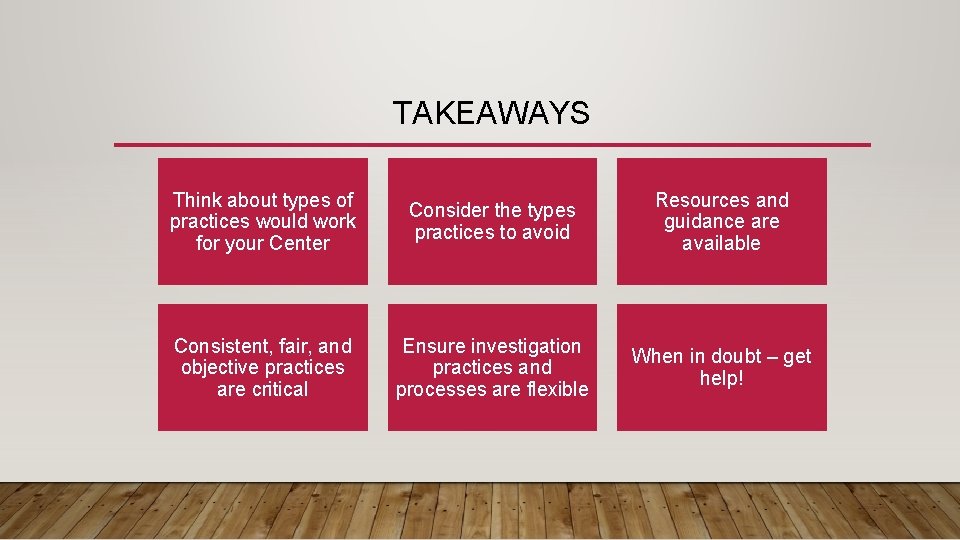
TAKEAWAYS Think about types of practices would work for your Center Consider the types practices to avoid Resources and guidance are available Consistent, fair, and objective practices are critical Ensure investigation practices and processes are flexible When in doubt – get help!

DISCLAIMER Any statements, recommendations, and opinions made by the Presenters in and during this presentation are our own, and do not belong to our respective Centers or otherwise are not reflective of each of our Centers’ stance, position, or reputation. This presentation is meant to serve as education and guidance tool, and not meant to be construed as professional or legal advice.

REFERENCES • Links • “Measuring Compliance Program Effectiveness: A Resource Guide”, March 27, 2017 • https: //oig. hhs. gov/compliance-resource-portal/files/HCCA-OIG-Resource-Guide. pdf • U. S. Office of Inspector General Website • https: //oig. hhs. gov • United States ex rel. Baklid Kunz v. Halifax Hospital Medical Center • https: //www. duanemorris. com/site/static/United_States_ex_rel. _Baklid. Kunz_v. _Halifax_Hospital_Medical_Center. pdf • Health Care Compliance Association Website • https: //www. hcca-info. org

QUESTIONS Donna Moore Director of Quality Management (936)558 -6232 donna. moore@myburke. org Rene Navarro, MPA, CHP Chief Compliance Officer (915)887 -3410 ext. 18819 rnavarro@ehnelpaso. org Rik Lindahl Saroeun Svay Director, Compliance, Planning Compliance Manager and Quality Assurance (214) 743 -6189 (972) 562 -0190 ext. 6112 rlindahl@lifepathsystems. org saroeun. svay@metrocareservices. org Ratana De. Luca Chief Compliance Officer (214) 743 -1223 ratana. deluca@metrocareservices. org