HOW SCIENTISTS WORK PARTS OF AN EXPERIMENT About

HOW SCIENTISTS WORK

PARTS OF AN EXPERIMENT About 400 years ago, people began to use experiments to answer their questions about life. Whenever possible a hypothesis should be tested by an experiment in which only one variable is changed at a time. All other variables should be kept unchanged, or controlled. • The variable that is deliberately changed is call the independent variable. • The variable that is observed and that changes in response to the independent variable is called the dependent variable. • A control is a factor of the experiment that the scientists purposely keeps the same

SPONTANEOUS GENERATION For many years observations seemed to indicate that some living things could just suddenly appear; maggots showed up on meat; mice were found on grain’ and beetles turned up on cow dung. People wondered how these events happened. For centuries people accepted that life somehow “arose” from nonliving matter. Scholars named this Spontaneous Generation: the idea that life can arise from nonliving things.

FRANCESCO REDI In 1668, Redi proposed a different hypothesis for the appearance of maggots. Redi had observed that these organisms appeared on meat a few days after flies were present. He considered it likely that the flies laid eggs too small for people to see. So he produced a new hypothesis—flies produce maggots. His next step was to test his hypothesis. Redi made a prediction that keeping flies away from meat would prevent the appearance of maggots. To test his hypothesis he planned the experiment shown below. He controlled all variables except whether or not there was gauze over each jar (the gauze kept the flies off the meat).

JOHN NEEDHAM In the mid 1700 s, Needham used an experiment involving animalcules to attack Redi’s work. Needham claimed that spontaneous generation could occur under the right conditions. To prove his claim, he sealed a bottle of gravy and heated it. He claimed that the heat had killed any living things that might be in the gravy. After several days, he examined the contents of the bottle and found it swarming with activity. He inferred that these “little animals” could have only come from the gravy juice.

LAZZARO SPALLANZANI Spallanzani read about Redi’s and Needham’s work. Spallanzani thought that Needham had not heated his samples enough and decided to improve upon Needham’s experiment. Spallanzani boiled 2 containers of gravy, assuming the boiling would kill any living things that were present. He sealed one jar immediately and left the other jar open. After a few days the gravy in the open jar was teeming with microorganisms. The sealed jar remained free of microorganisms. He concluded that nonliving gravy did not produce living things. The microorganisms in the unsealed jar were offspring of the microorganisms that had entered the jar through the air.

LOUIS PASTEUR 1864 Pasteur designed a flask that had a long curved neck. The flask remained open to the air, but microorganisms from the air did not make their way through the neck into the flask. He was able to show that as long as the broth was protected from microorganisms, it remained free of living things. A year after his experiment the neck of the flask was broken and the broth quickly filled with microorganisms. His work showed that all living things come from other living things.

THEORIES When evidence from numerous investigations builds up, a particular hypothesis (a proposed explanation for a phenomenon) may become so well supported that scientists consider it a theory- a well tested explanation that unifies a broad range of observations. Ex. The hypothesis that new organisms come from existing organisms— now known as biogenesis, meaning “generating from life” Theory of Evolution- various types of animals and plants have their origin in other preexisting types and that the distinguishable differences are due to modifications in successive generations Big Bang Theory- About 15 billion years ago a tremendous explosion started the expansion of the universe. This explosion is known as the Big Bang. At the point of this event all of the matter and energy of space was contained at one point.
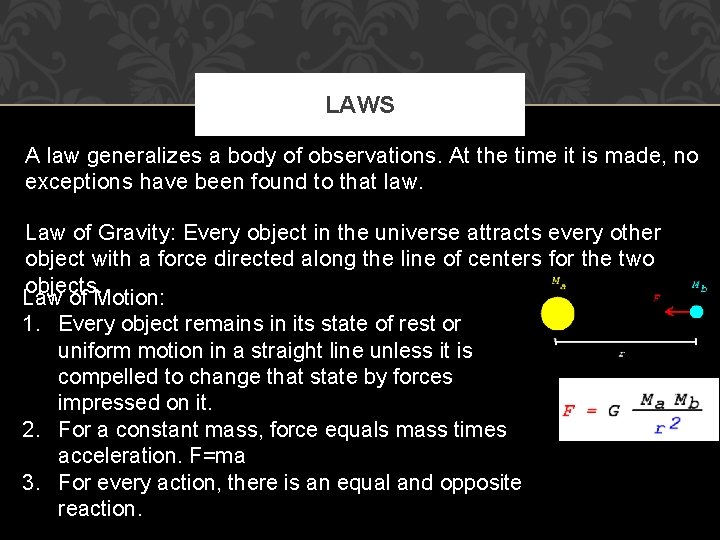
LAWS A law generalizes a body of observations. At the time it is made, no exceptions have been found to that law. Law of Gravity: Every object in the universe attracts every other object with a force directed along the line of centers for the two objects. Law of Motion: 1. Every object remains in its state of rest or uniform motion in a straight line unless it is compelled to change that state by forces impressed on it. 2. For a constant mass, force equals mass times acceleration. F=ma 3. For every action, there is an equal and opposite reaction.
BASIC CHEMISTRY OF LIFE Atoms-the basic unit of matter v The subatomic particles that make up atoms are protons, neutrons, and electrons. v Protons are (+), Electrons are (-), and Neutrons carry no charge. v Strong forces bring protons and neutrons together to form the nucleus of the atom. Elements-a pure substance that consists entirely of one type of atom. v Elements are represented by a one or two letter symbol. v Ex. C stands for carbon, H stands for hydrogen, and Na for sodium
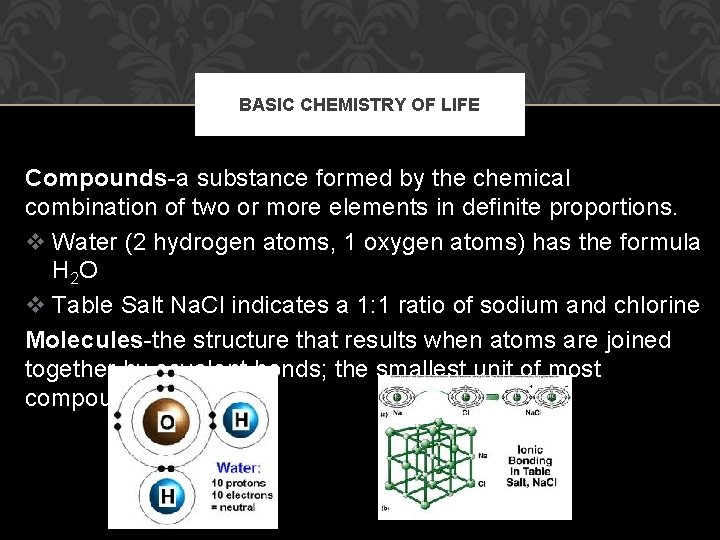
BASIC CHEMISTRY OF LIFE Compounds-a substance formed by the chemical combination of two or more elements in definite proportions. v Water (2 hydrogen atoms, 1 oxygen atoms) has the formula H 2 O v Table Salt Na. Cl indicates a 1: 1 ratio of sodium and chlorine Molecules-the structure that results when atoms are joined together by covalent bonds; the smallest unit of most compounds.
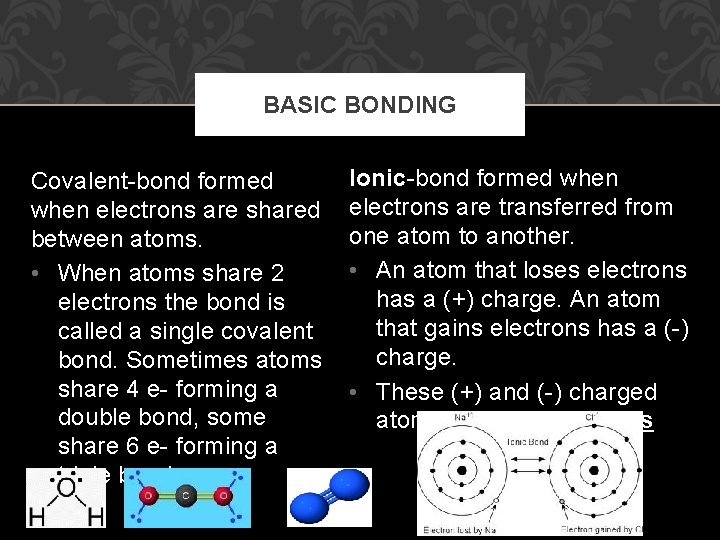
BASIC BONDING Covalent-bond formed when electrons are shared between atoms. • When atoms share 2 electrons the bond is called a single covalent bond. Sometimes atoms share 4 e- forming a double bond, some share 6 e- forming a triple bond. Ionic-bond formed when electrons are transferred from one atom to another. • An atom that loses electrons has a (+) charge. An atom that gains electrons has a (-) charge. • These (+) and (-) charged atoms are known as ions
- Slides: 12