How PVL Post TAVR Impact Outcome And How












- Slides: 36

How PVL Post TAVR Impact Outcome And How To Minimize It Dr Ganesh Manoharan MBBch, MD, FRCP(I), FRCP(Edin) Consultant Cardiologist Royal Victoria Hospital Belfast

Disclosure Statement of Financial Interest Within the past 12 months, I or my spouse/partner have had a financial interest/arrangement or affiliation with the organization(s) listed below. • Consulting Fees/Honoraria • Boston Scientific • Medtronic Cardiovascular • St Judes Medical

Assessment of PVL • None is easy to assess • Severe should be easy to assess as well • Can be challenging to differentiate between mild and moderate • Difficulty arises when multiple jets are assessed: echo may underestimate true volume of regurgitant flow MRI superior 1 (but not practical) 1. Sherif MA; Euro. Intervention. 2011; 7: 57– 63

Procedural Assessment of PVL: Overview • Echo – TTE or TEE • Aortography – Pigtail size and position – Volume of contrast – Pressure of injection • Pressure – Diacrotic notch – Simultaneous LV and aortic pressure assessment: AR Index – Aortic diastolic pressure pre and post TAVR

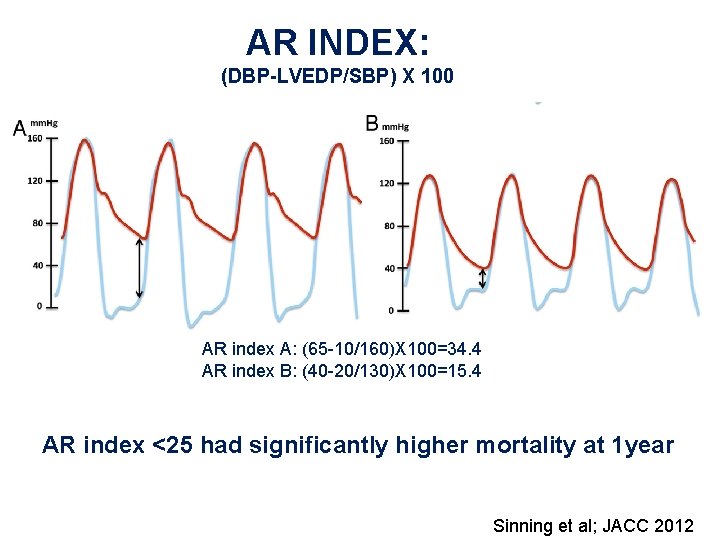
AR INDEX: (DBP-LVEDP/SBP) X 100 AR index A: (65 -10/160)X 100=34. 4 AR index B: (40 -20/130)X 100=15. 4 AR index <25 had significantly higher mortality at 1 year Sinning et al; JACC 2012

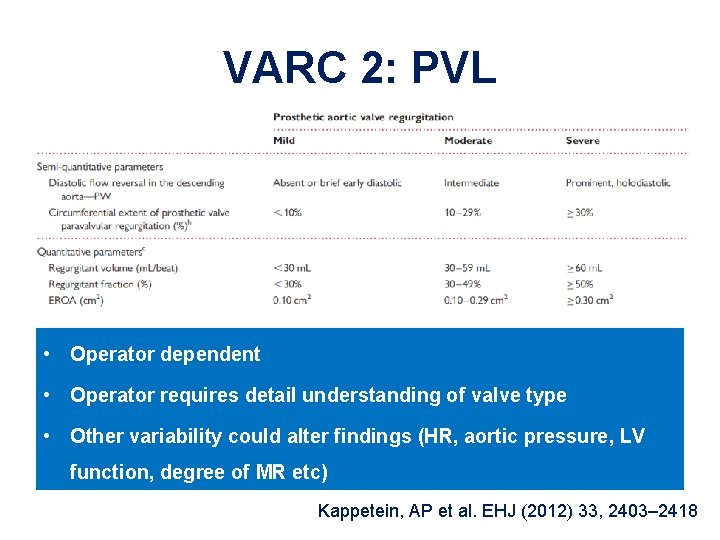
VARC 2: PVL • Operator dependent • Operator requires detail understanding of valve type • Other variability could alter findings (HR, aortic pressure, LV function, degree of MR etc) Kappetein, AP et al. EHJ (2012) 33, 2403– 2418

Assessment of PVL: Summary • • No method is absolutely accurate None = none Nothing more to do Severe = severe Have to fix it If borderline, then use all modalities

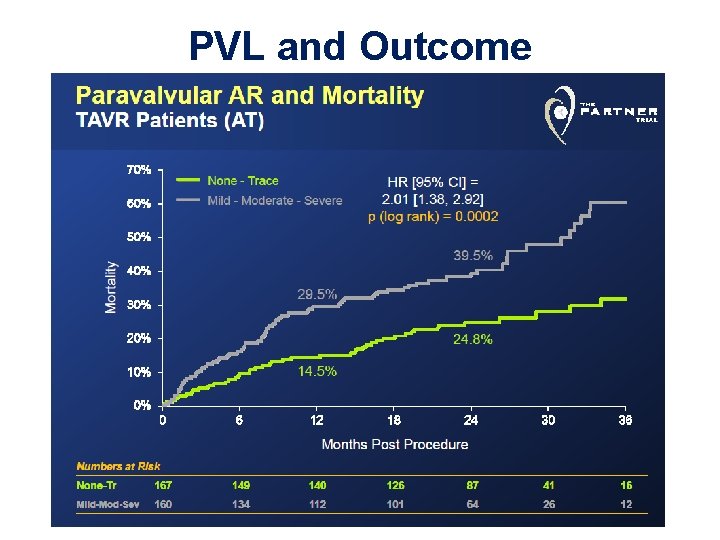
PVL and Outcome

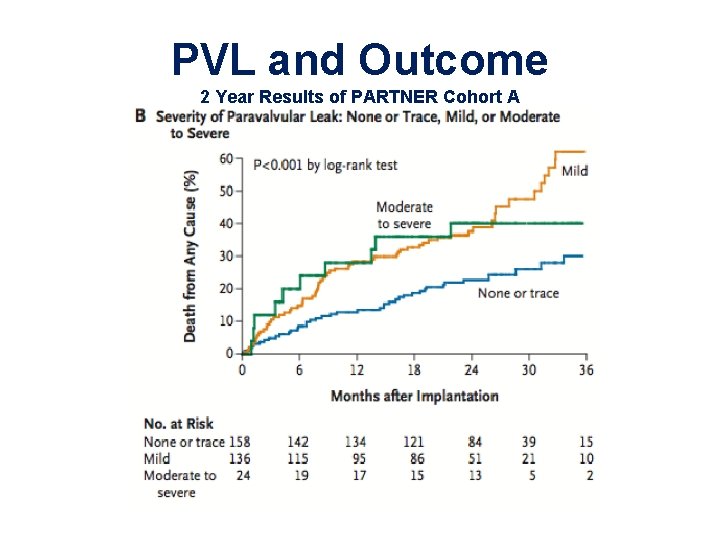
PVL and Outcome 2 Year Results of PARTNER Cohort A

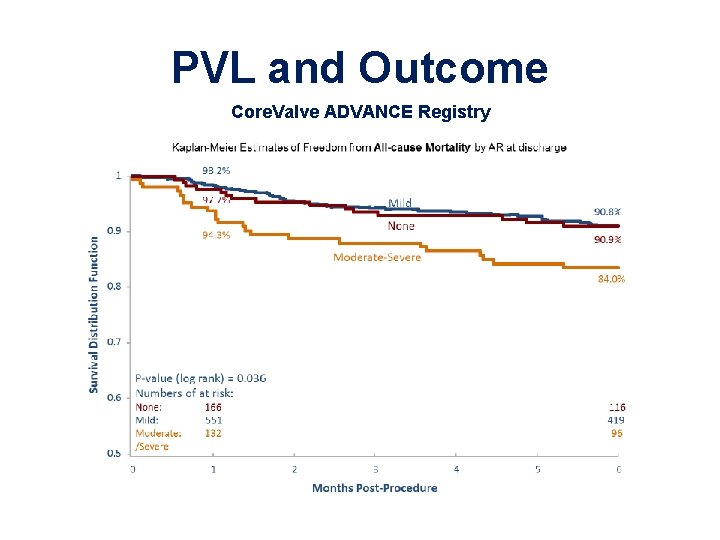
PVL and Outcome Core. Valve ADVANCE Registry

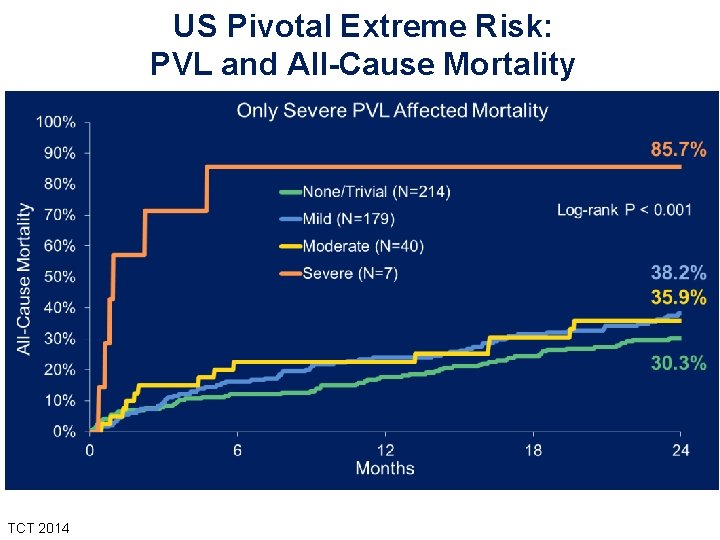
US Pivotal Extreme Risk: PVL and All-Cause Mortality TCT 2014

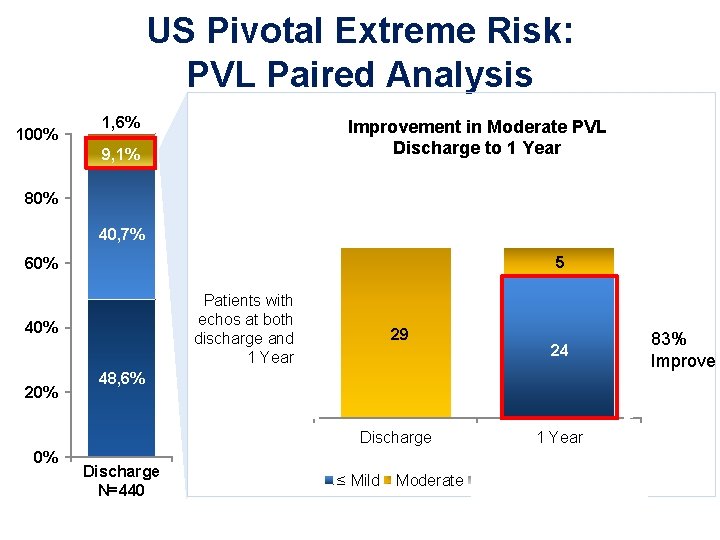
US Pivotal Extreme Risk: PVL Paired Analysis 100% 1, 6% Improvement in Moderate PVL Discharge to 1 Year 9, 1% Percentage of Patients 80% 40, 7% 2 Those who died or had no echo at 1 year 11 9 5 60% Patients with echos at both discharge and 1 Year 40% 29 24 48, 6% Discharge 0% Discharge N=440 ≤ Mild Moderate 1 Year Died No 1 Yr Echo 83% Improve

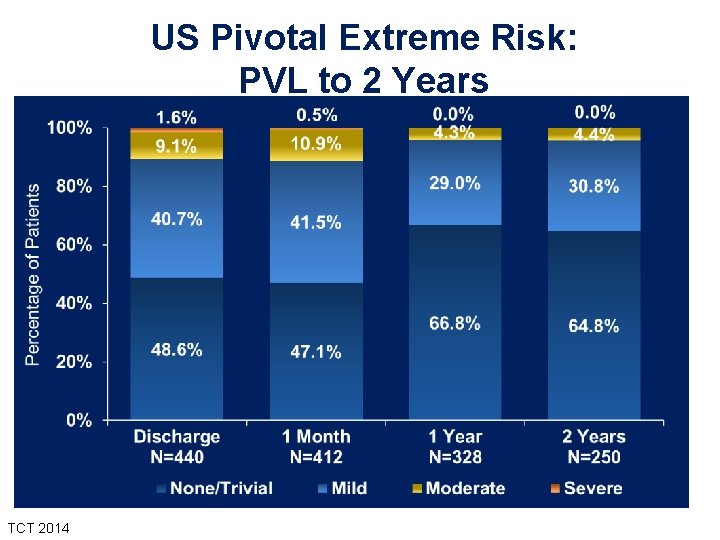
US Pivotal Extreme Risk: PVL to 2 Years TCT 2014

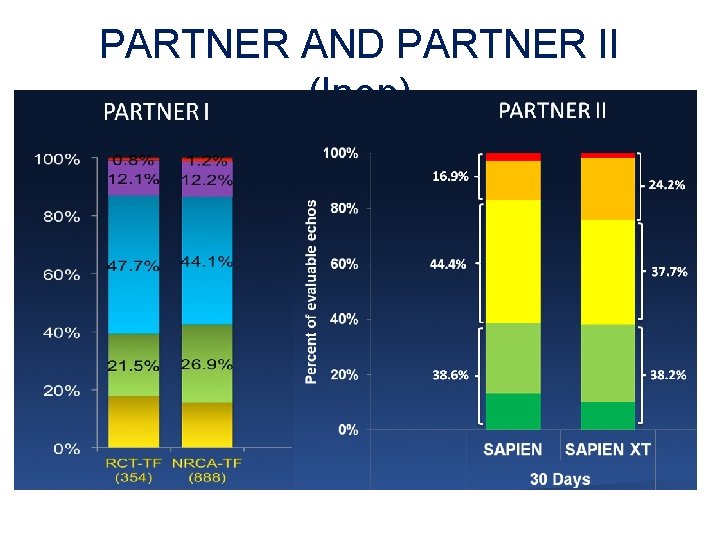
PARTNER AND PARTNER II (Inop)

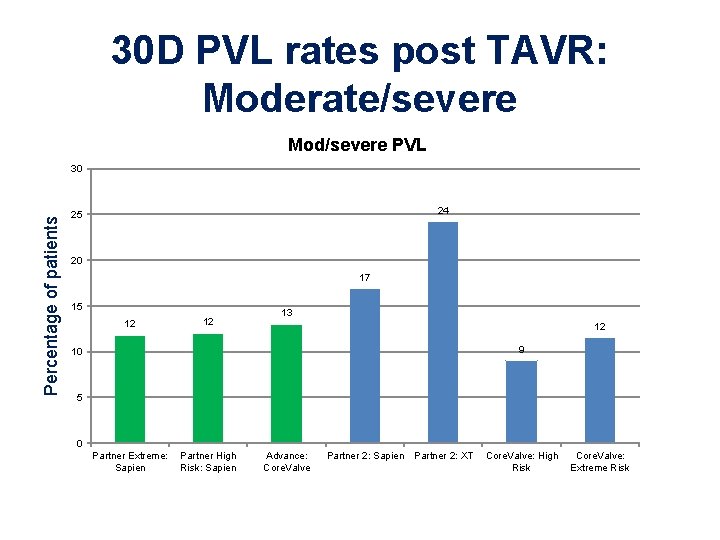
30 D PVL rates post TAVR: Moderate/severe Mod/severe PVL Percentage of patients 30 24 25 20 17 15 12 12 13 12 9 10 5 0 Partner Extreme: Sapien Partner High Risk: Sapien Advance: Core. Valve Partner 2: Sapien Partner 2: XT Core. Valve: High Risk Core. Valve: Extreme Risk

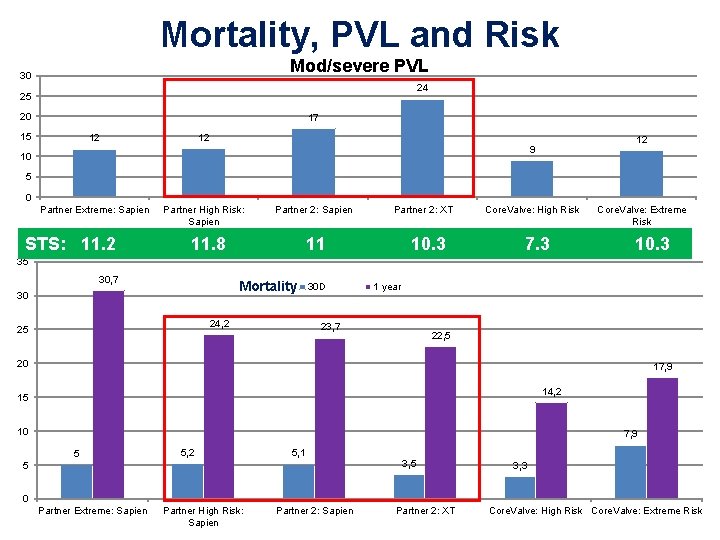
Mortality, PVL and Risk Mod/severe PVL 30 24 25 20 17 15 12 12 12 9 10 5 0 Partner Extreme: Sapien STS: 11. 2 Partner High Risk: Sapien Partner 2: Sapien 11. 8 Partner 2: XT 11 10. 3 Core. Valve: High Risk 7. 3 Core. Valve: Extreme Risk 10. 3 35 30, 7 Mortality 30 24, 2 25 30 D 1 year 23, 7 22, 5 20 17, 9 14, 2 15 10 7, 9 5 5, 2 5, 1 5 3, 3 0 Partner Extreme: Sapien Partner High Risk: Sapien Partner 2: XT Core. Valve: High Risk Core. Valve: Extreme Risk

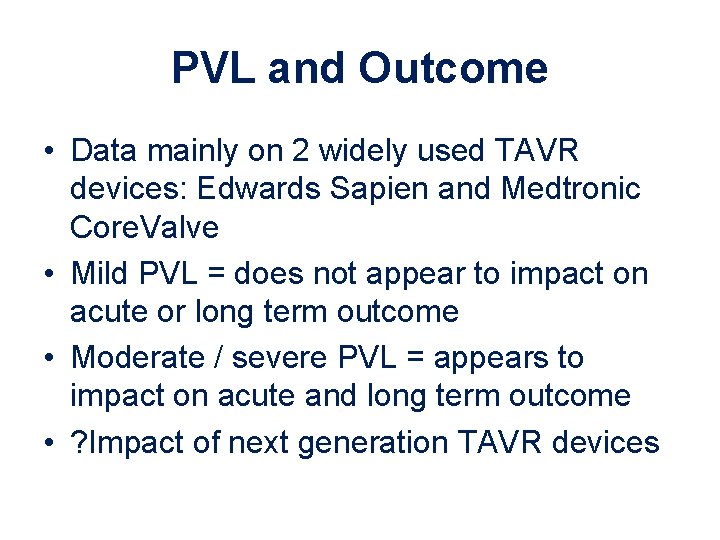
PVL and Outcome • Data mainly on 2 widely used TAVR devices: Edwards Sapien and Medtronic Core. Valve • Mild PVL = does not appear to impact on acute or long term outcome • Moderate / severe PVL = appears to impact on acute and long term outcome • ? Impact of next generation TAVR devices

How to Minimize Risk of PVL

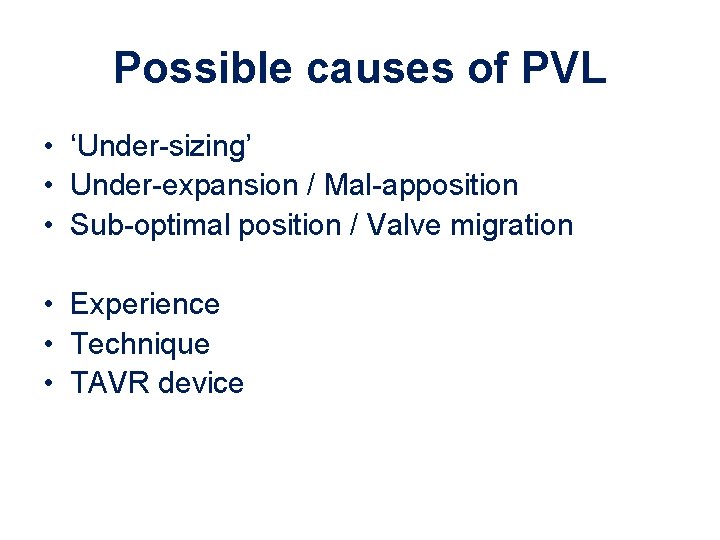
Possible causes of PVL • ‘Under-sizing’ • Under-expansion / Mal-apposition • Sub-optimal position / Valve migration • Experience • Technique • TAVR device

‘Under-sizing’ = misnomer • If the valve is under-sized, it will float out • Appropriate over-sizing is required to minimise PVL – Usually 10 to 15% is required for valve stability and seal – Care with Edwards = significant over-sizing can cause annular rupture – Care with annular self-expanding = significant over-sizing can cause leaftlet malfunction

Optimising valve sizing • Use MSCT – Learning curve – Dedicated staff/team – Protocols for AF patients – Risk of contrast nephropathy • 3 D TEE – Learning curve – Dedicated staff/team – May not be tolerated by some patients • Ideally, expertise in both techniques should be available in a TAVR centre

Under-expansion / Malapposition • Calcification – More of a problem with self-expanding, but, – Increased risk of ruptured with balloon expandable • Elliptical annulus – More of a problem with balloon expandable • Overall, more common with self-expanding systems

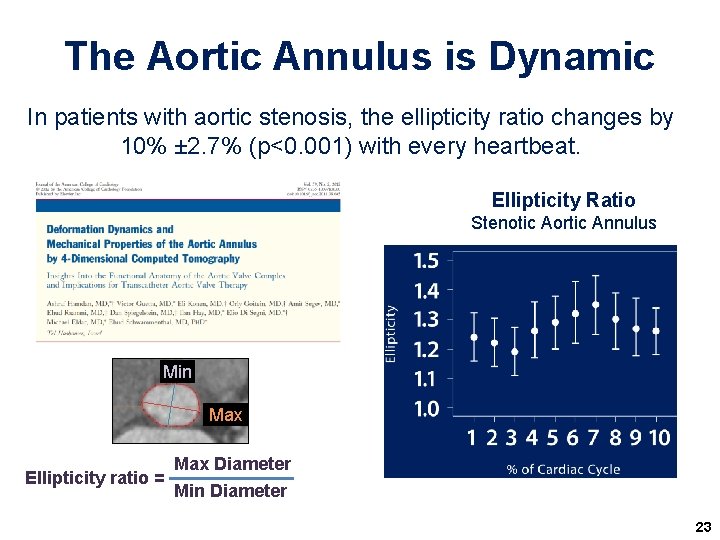
The Aortic Annulus is Dynamic In patients with aortic stenosis, the ellipticity ratio changes by 10% ± 2. 7% (p<0. 001) with every heartbeat. Ellipticity Ratio Stenotic Aortic Annulus Min Max Ellipticity ratio = Max Diameter Min Diameter 23

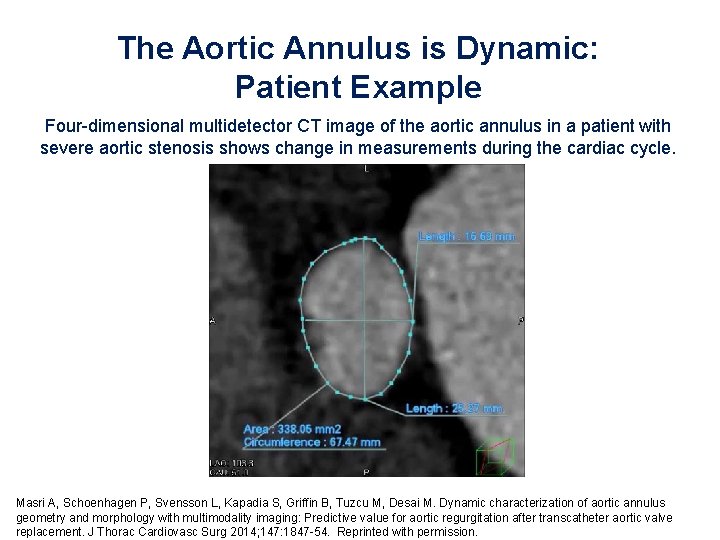
The Aortic Annulus is Dynamic: Patient Example Four-dimensional multidetector CT image of the aortic annulus in a patient with severe aortic stenosis shows change in measurements during the cardiac cycle. Masri A, Schoenhagen P, Svensson L, Kapadia S, Griffin B, Tuzcu M, Desai M. Dynamic characterization of aortic annulus geometry and morphology with multimodality imaging: Predictive value for aortic regurgitation after transcatheter aortic valve replacement. J Thorac Cardiovasc Surg 2014; 147: 1847 -54. Reprinted with permission.

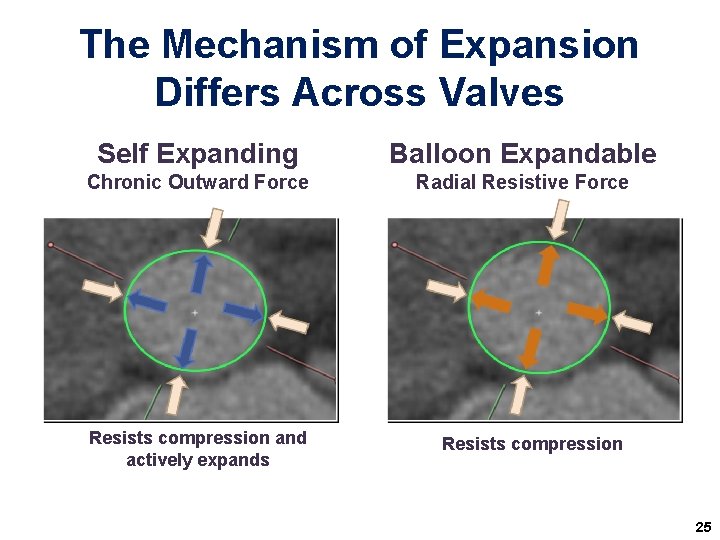
The Mechanism of Expansion Differs Across Valves Self Expanding Balloon Expandable Chronic Outward Force Radial Resistive Force Resists compression and actively expands Resists compression 25

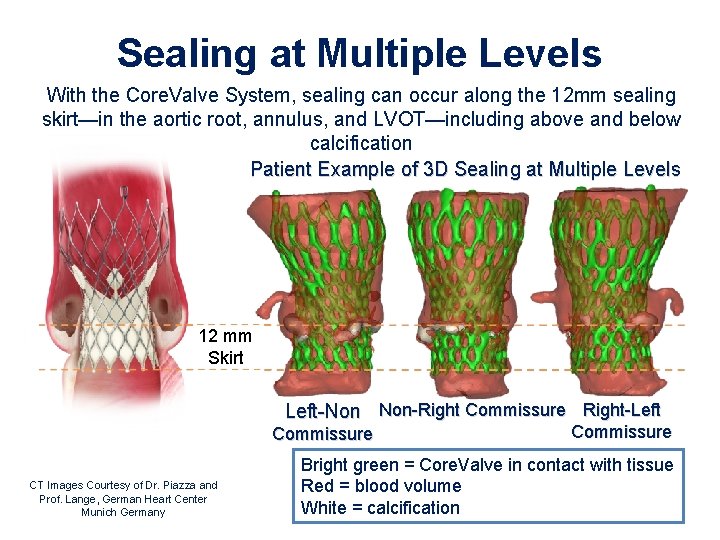
Sealing at Multiple Levels With the Core. Valve System, sealing can occur along the 12 mm sealing skirt—in the aortic root, annulus, and LVOT—including above and below calcification Patient Example of 3 D Sealing at Multiple Levels 12 mm Skirt Left-Non Non-Right Commissure Right-Left Commissure CT Images Courtesy of Dr. Piazza and Prof. Lange, German Heart Center Munich Germany Commissure Bright green = Core. Valve in contact with tissue Red = blood volume White = calcification

Calcification impacts on outcome John et al, JACC interv, 2010 Stauback et al, CCVI, 2013

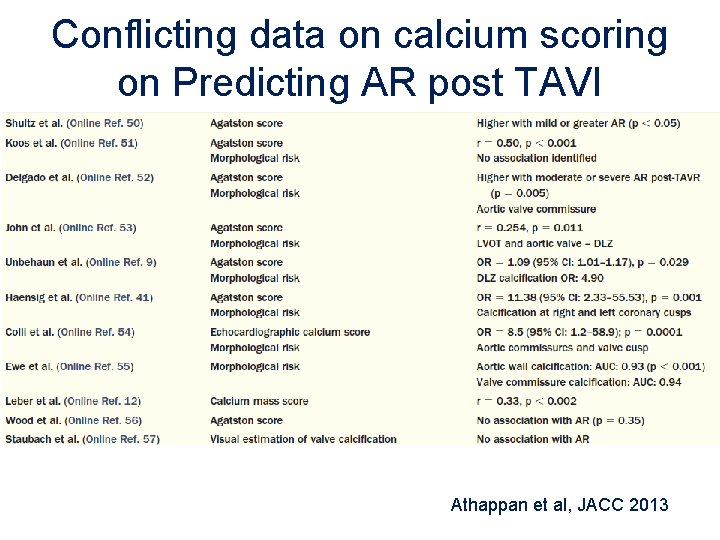
Conflicting data on calcium scoring on Predicting AR post TAVI Athappan et al, JACC 2013

Calcification impacts on outcome • Volume of calcification impact on outcome post TAVI – More so with balloon expandable device (Watanabe, TCT 2013) • Distribution has potential to impact on outcome • But outcome can still be variable – Device type? – Depth of implant? – Predilatation strategy?

How to deal with it underexpansion/mal-apposition? • Post dilatation – – Risk of CVA Risk of annular rupture Risk of coronary occlusion Risk of valve migration = worsening PVL • TAVR in TAVR (Tin. T) – Risk of migration of 1 st valve – Risk of coronary occlusion – Risk of annular rupture (Sapien) • Reconsider AVR

Valve sub-optimal positioning • • • Learning curve / technique – new device Valve size mismatch Sub-optimal wire control Sub-optimal valve release/deployment – too fast Sub-optimal valve fixation – – Low degree of annular calcification: Aortic regurgitation – Degree of valve over-sizing • Hyperdynamic circulation • Severe septal hypertrophy/septal bulge

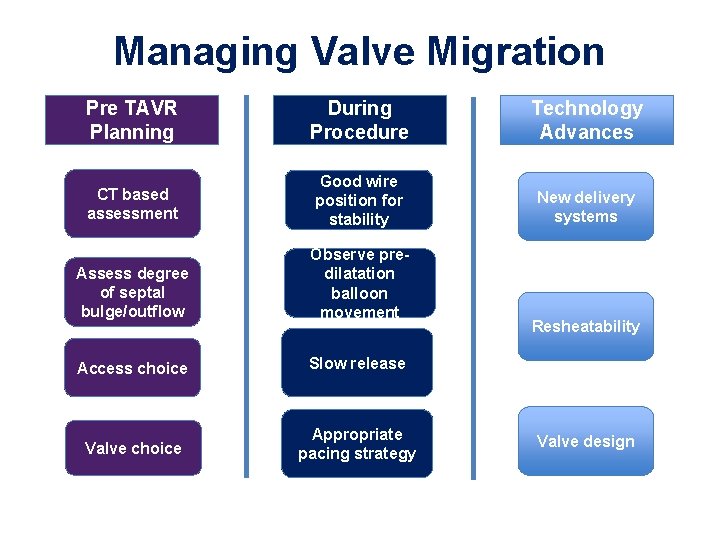
Managing Valvular Migration

Managing Valve Migration Pre TAVR Planning During Procedure Technology Advances CT based assessment Good wire position for stability New delivery systems Assess degree of septal bulge/outflow Observe predilatation balloon movement Access choice Slow release Valve choice Appropriate pacing strategy Resheatability Valve design

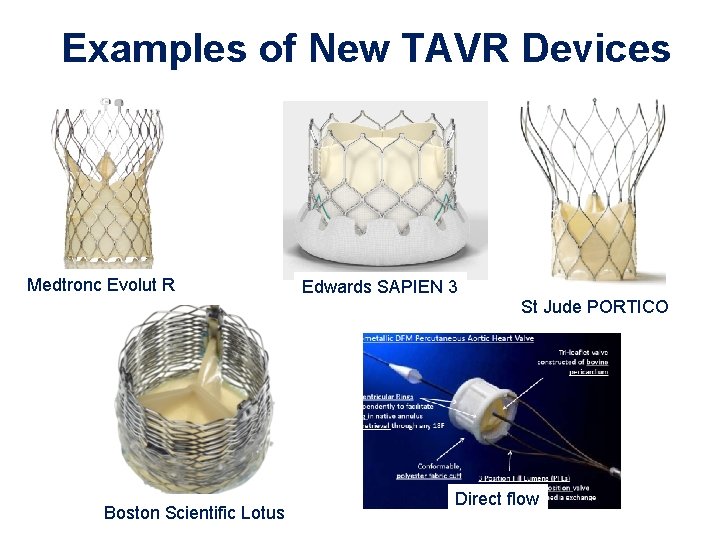
Examples of New TAVR Devices Medtronc Evolut R Edwards SAPIEN 3 St Jude PORTICO Boston Scientific Lotus Direct flow

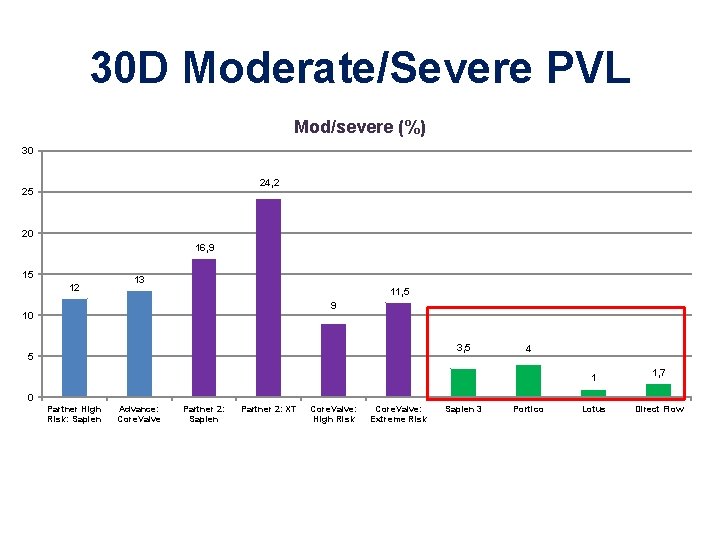
30 D Moderate/Severe PVL Mod/severe (%) 30 24, 2 25 20 16, 9 15 12 13 11, 5 9 10 3, 5 5 4 1 1, 7 0 Partner High Risk: Sapien Advance: Core. Valve Partner 2: Sapien Partner 2: XT Core. Valve: High Risk Core. Valve: Extreme Risk Sapien 3 Portico Lotus Direct Flow

To conclude • We have standardised how to report but not how to assess PVL post TAVR • Moderate / severe PVL results in poor outcome • Pre-procedural sizing and adherence to best practice reduces PVL rates • Patient selection will improve PVL • Next generation devices, with repositionability, dedicated skirt and conformable frame will further mitigate PVL post TAVR