How NITAG work is documented Best practices Reviewed

How NITAG work is documented: Best practices Reviewed by WHO and partners The content for these slides was developed by Kathy Cavallaro, and they are used with her kind permission.

Overview • • Minutes of meetings Policy Brief to Mo. H Publishing recommendations Dissemination of NITAG work to general public (websites)
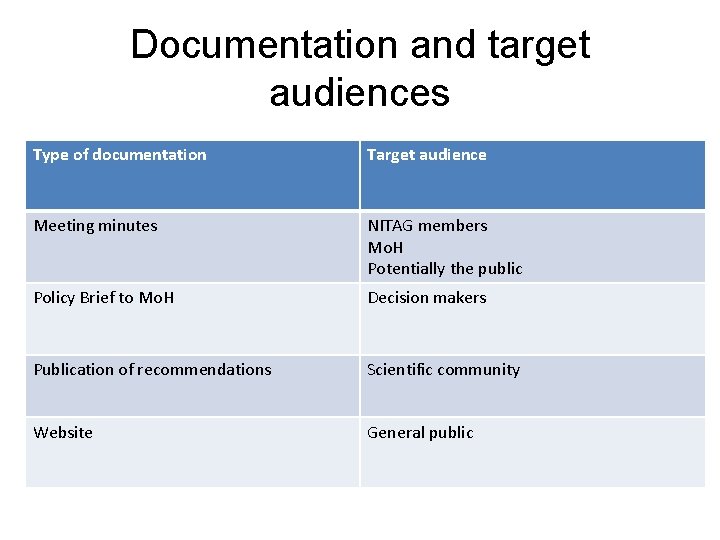
Documentation and target audiences Type of documentation Target audience Meeting minutes NITAG members Mo. H Potentially the public Policy Brief to Mo. H Decision makers Publication of recommendations Scientific community Website General public

Meeting minutes Who is it for? • Internal use within NITAG Purpose • Provide a tangible record of the meeting • Document the deliberation process, and the decisions taken, with rationale • Track next steps and action items

What should minutes include? • Date and time of the meeting • Names of participants, chair, and members unable to attend, and whether quorum was met • Acceptance/corrections to previous meeting minutes • List of agenda items or topics • Summary of discussion for each agenda item, not verbatim, using a standard format • Actions taken or agreed to be taken • Next meeting date and time

Meeting minutes: country example France https: //www. has-sante. fr/portail/upload/docs/application/pdf/201903/proces_verbal_ctv_22_janvier_2019. pdf
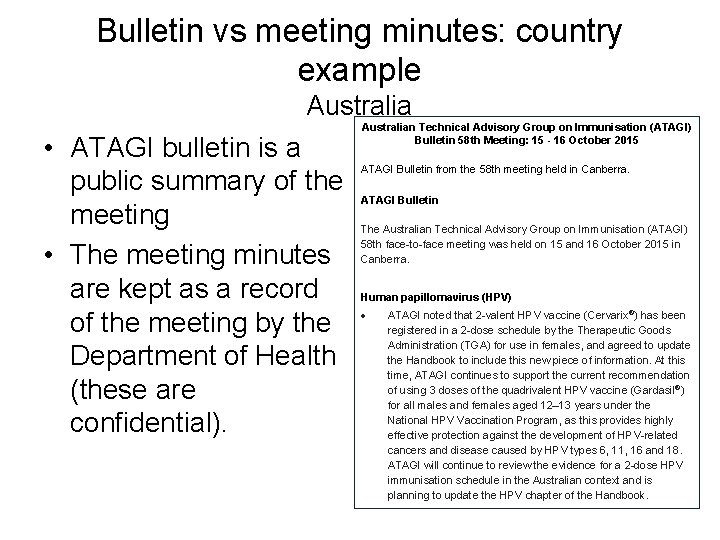
Bulletin vs meeting minutes: country example Australia • ATAGI bulletin is a public summary of the meeting • The meeting minutes are kept as a record of the meeting by the Department of Health (these are confidential). Australian Technical Advisory Group on Immunisation (ATAGI) Bulletin 58 th Meeting: 15 - 16 October 2015 ATAGI Bulletin from the 58 th meeting held in Canberra. ATAGI Bulletin The Australian Technical Advisory Group on Immunisation (ATAGI) 58 th face-to-face meeting was held on 15 and 16 October 2015 in Canberra. Human papillomavirus (HPV) ATAGI noted that 2 -valent HPV vaccine (Cervarix®) has been registered in a 2 -dose schedule by the Therapeutic Goods Administration (TGA) for use in females, and agreed to update the Handbook to include this new piece of information. At this time, ATAGI continues to support the current recommendation of using 3 doses of the quadrivalent HPV vaccine (Gardasil®) for all males and females aged 12– 13 years under the National HPV Vaccination Program, as this provides highly effective protection against the development of HPV-related cancers and disease caused by HPV types 6, 11, 16 and 18. ATAGI will continue to review the evidence for a 2 -dose HPV immunisation schedule in the Australian context and is planning to update the HPV chapter of the Handbook.

Policy Brief • Target audience: Mo. H decision-makers • Purpose: – Provide a summary of the evidence supporting the recommendation, rationale for decision – Support decision-making • Format: Consistent, clear, logical flow, short, ideally less than 2 -4 pages (1, 500 words)

Policy Brief, template Section headings • Introduction including policy question from Mo. H • Method for evidence search • Disease burden • Vaccine efficacy and risk of serious complications • Benefits versus risks • Cost-effectiveness of vaccination • Recommended vaccine strategy specifying vaccine, age groups, number of doses, schedule • Implementation aspects

Policy Brief, content (1) • Introduction – What is the policy question? – Who added the topic to the NITAG agenda? • Was it in the annual work plan, or suggested by the Ministry of Health or a NITAG member? – Why was the topic added to the agenda? • Opportunity of introduction of a new vaccine, ongoing epidemic, new data on vaccine efficacy or disease burden? – Brief description of the problem identified • Methods – Describe the evidence search process

Policy Brief, content (2) • Disease burden, vaccine efficacy, benefits vs risks, costeffectiveness – Findings on these topics and other criteria in the recommendation framework obtained from literature or systematic reviews, surveillance data, programmatic information. – Discussion of the evidence • Recommended vaccine strategy – Proposed recommendations clearly formulated and linked to the available evidence – Specifics on vaccine, age groups, number of doses, schedule • Implementation considerations – Barriers to implementation and potential solutions to overcome them – Efficiency, acceptability, equity, feasibility, sustainability aspects

Policy Brief, Best practice Do’s Is recommended Is not recommended Policy-makers should Should not Don’t We suggest Policy-makers might The language used to convey recommendations should be unambiguous and only strong recommendations are provided.

Policy Brief, country example: Recommendations on rotavirus vaccine, Netherlands Section headings • Introduction including policy question from Mo. H • Disease burden due to rotavirus considerable • Slight risk of serious complications • Benefits outweighs risk • Vaccination is not cost-effective at current vaccine prices • Recommendation vaccine strategy • Implementation aspects Length • 5 pages (996 words)

Policy Brief, country example: Recommendations on rotavirus vaccine, Netherlands

Publication of recommendations • Target audience: Scientific community, researchers • Purpose: – Provides technical details and rationale for decision – Lends credibility to recommendations • Format: depends on journal • Content – What does the scientific community need to know? • Vaccine, target population, age, doses, schedule, delivery strategy, AEFI surveillance • Technical rationale
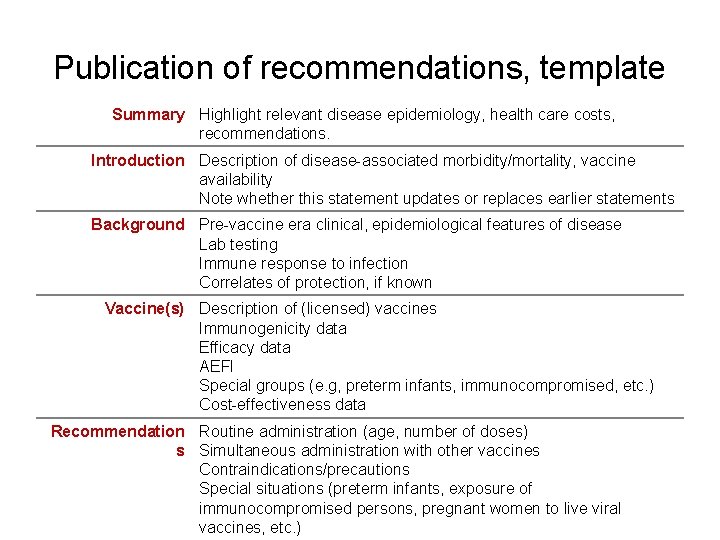
Publication of recommendations, template Summary Highlight relevant disease epidemiology, health care costs, recommendations. Introduction Description of disease-associated morbidity/mortality, vaccine availability Note whether this statement updates or replaces earlier statements Background Pre-vaccine era clinical, epidemiological features of disease Lab testing Immune response to infection Correlates of protection, if known Vaccine(s) Description of (licensed) vaccines Immunogenicity data Efficacy data AEFI Special groups (e. g, preterm infants, immunocompromised, etc. ) Cost-effectiveness data Recommendation Routine administration (age, number of doses) s Simultaneous administration with other vaccines Contraindications/precautions Special situations (preterm infants, exposure of immunocompromised persons, pregnant women to live viral vaccines, etc. )

Publications of recommendations, country example: Outline of recommendations on HPV, USA Section headings • Introduction • Background • Methods • Summary of Key Findings • Rationale • Recommendations – including routine and catch-up age groups; dosing schedule; persons previously vaccinated; interrupted schedules; special populations; medical conditions; contraindications and precautions • References Length • 3 pages, not including References

Publications of recommendations, country example: Recommendations on HPV, USA

Publications of recommendations, country example: Recommendations on inactivated zoster subunit vaccine, Germany https: //link. springer. com/content/pdf/10. 1007%2 Fs 00103 -019 -02882 -5. pdf

Websites for the general public, country examples • Argentina Co. Na. In https: //www. argentina. gob. ar/salud/inmunoprevenibles/comisiones/conain – Mission, vision; updated regularly; core members, liaison members and ex-officio members; links to other committees • Australia ATAGI http: //www. mvec. vic. edu. au/immunisation-references/atagi-australian-technical-advisory-group-onimmunisation/ – Membership guidelines with the Conflict of Interest procedures – Maintains a mailing list; sends minutes or recommendations upon request • France https: //www. has-sante. fr/portail/jcms/c_2755844/en/commission-technique-des-vaccinations – Links to legislative documents • Germany STIKO https: //www. rki. de/EN/Content/infections/Vaccination_node. html – Posts recommendations by disease
- Slides: 21