How Long Is The Hydrocarbon How long is
How Long Is The Hydrocarbon?

How long is the hydrocarbon? In this activity, you constructed models of hydrocarbons and compared their boiling points and molecular masses.

Which elements are in hydrocarbons? Two elements, carbon and hydrogen, make up a hydrocarbon. The simplest group of hydrocarbons is the alkanes, Cn. H 2 n+2

What else do you know about alkanes? Methane, CH 4, is the smallest alkane. It is a gas at room temperature (25 o. C) because it boils at -162 o. C. Other gases are ethane, propane and butane.

Why is octane a liquid and methane a gas? The physical and chemical properties of hydrocarbons depend on… the number and arrangement of the carbon and hydrogen atoms in the molecules.
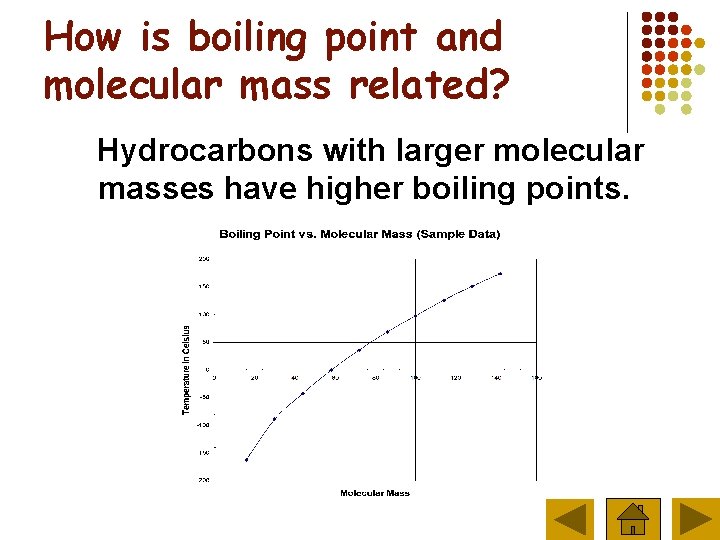
How is boiling point and molecular mass related? Hydrocarbons with larger molecular masses have higher boiling points.
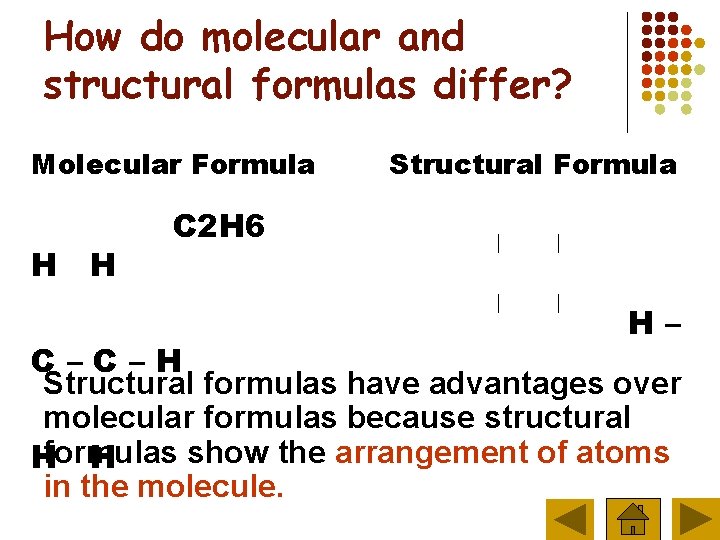
How do molecular and structural formulas differ? Molecular Formula H H Structural Formula C 2 H 6 H– C–C–H Structural formulas have advantages over molecular formulas because structural show the arrangement of atoms Hformulas H in the molecule.

Which alkanes are liquids at room temperature? Pentane Heptane Nonane Hexane Octane Decane
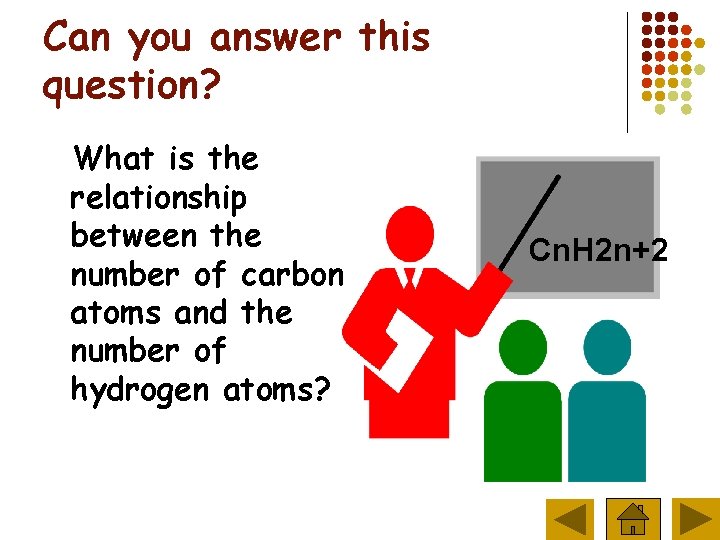
Can you answer this question? What is the relationship between the number of carbon atoms and the number of hydrogen atoms? Cn. H 2 n+2

Try this question! Why is natural gas (methane) or propane used for fueling our bunsen burners and heating our homes rather than octane?
- Slides: 10