How Ligand Properties Affect the Formation and Characteristics


- Slides: 23

How Ligand Properties Affect the Formation and Characteristics of Recoupled Pair Bonds Beth A. Lindquist, David E. Woon, and Thom H. Dunning 06/23/2011

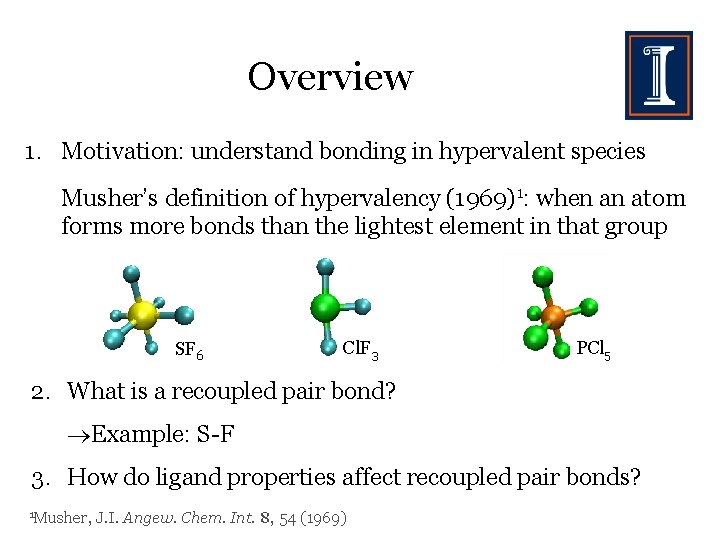
Overview 1. Motivation: understand bonding in hypervalent species Musher’s definition of hypervalency (1969)1: when an atom forms more bonds than the lightest element in that group SF 6 Cl. F 3 PCl 5 2. What is a recoupled pair bond? Example: S-F 3. How do ligand properties affect recoupled pair bonds? 1 Musher, J. I. Angew. Chem. Int. 8, 54 (1969)

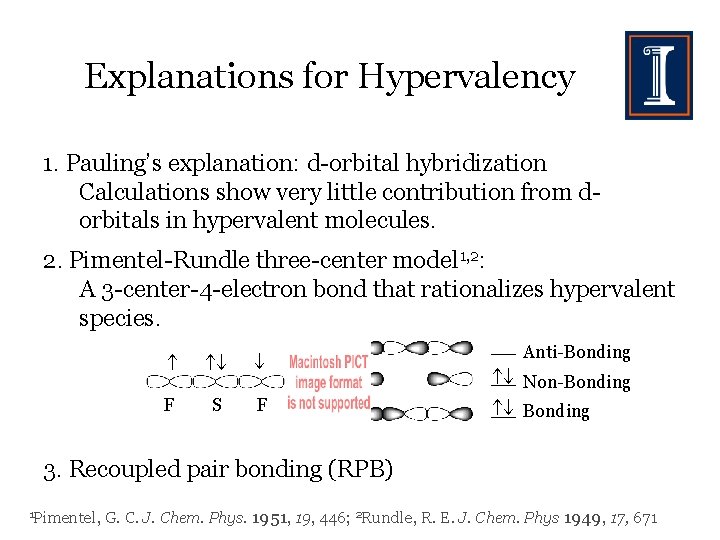
Explanations for Hypervalency 1. Pauling’s explanation: d-orbital hybridization Calculations show very little contribution from dorbitals in hypervalent molecules. 2. Pimentel-Rundle three-center model 1, 2: A 3 -center-4 -electron bond that rationalizes hypervalent species. F S F Anti-Bonding Non-Bonding 3. Recoupled pair bonding (RPB) 1 Pimentel, G. C. J. Chem. Phys. 1951, 19, 446; 2 Rundle, R. E. J. Chem. Phys 1949, 17, 671

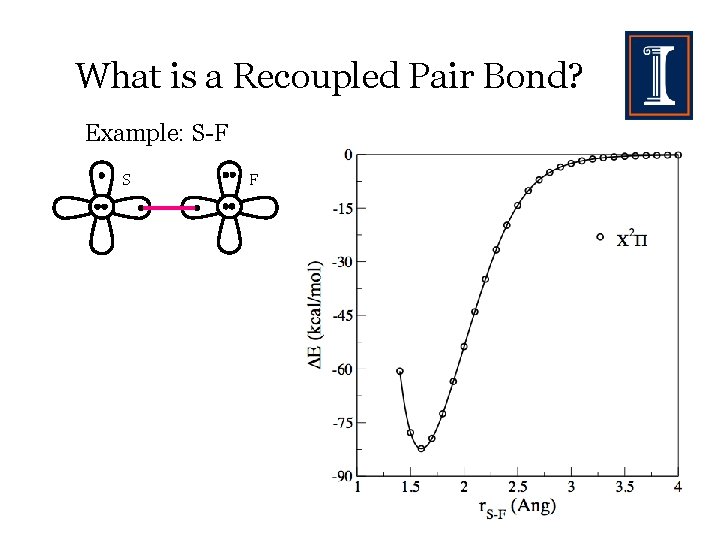
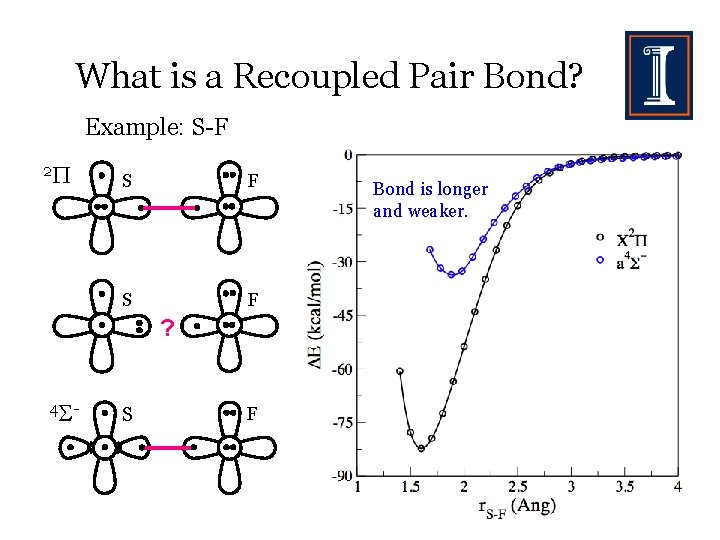
What is a Recoupled Pair Bond? Example: S-F S F

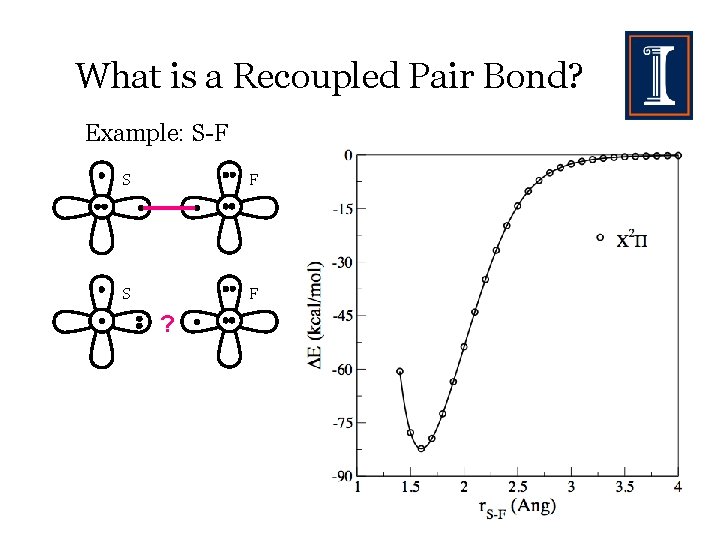
What is a Recoupled Pair Bond? Example: S-F S F ?

What is a Recoupled Pair Bond? Example: S-F 2 S F ? 4 - S F Bond is longer and weaker.

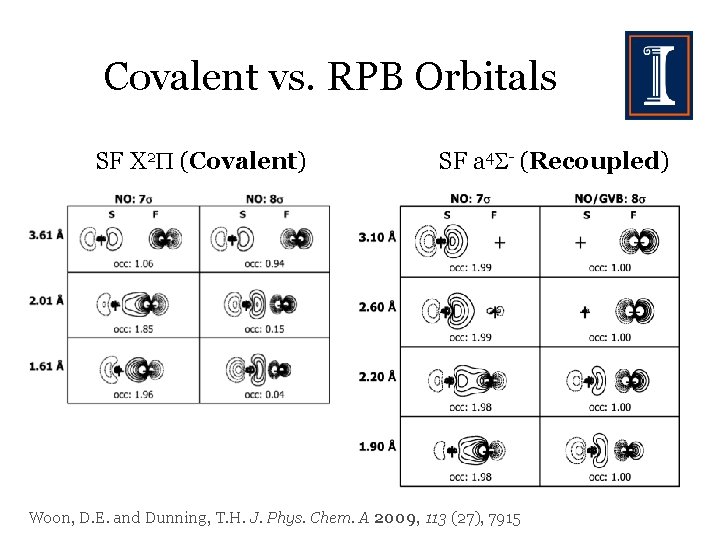
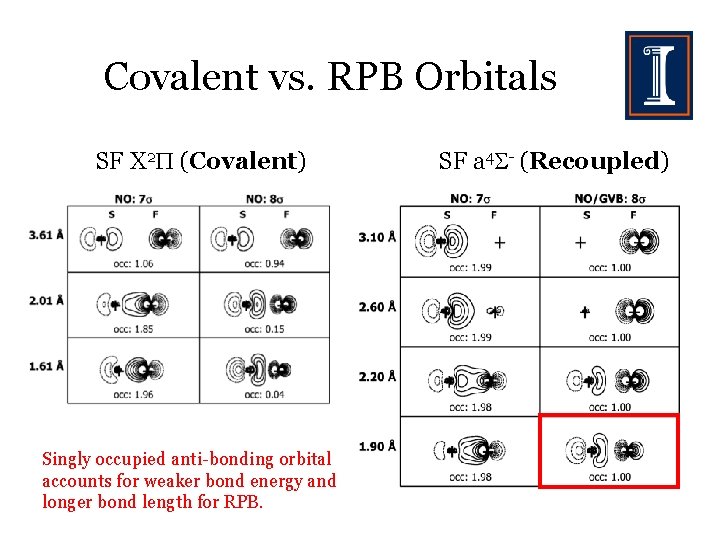
Covalent vs. RPB Orbitals SF X 2 (Covalent) SF a 4 - (Recoupled) Woon, D. E. and Dunning, T. H. J. Phys. Chem. A 2009, 113 (27), 7915

Covalent vs. RPB Orbitals SF X 2 (Covalent) Singly occupied anti-bonding orbital accounts for weaker bond energy and longer bond length for RPB. SF a 4 - (Recoupled)

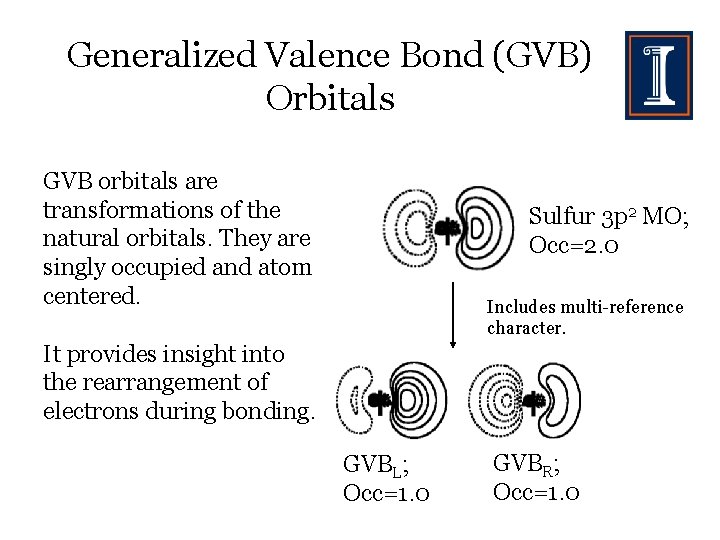
Generalized Valence Bond (GVB) Orbitals GVB orbitals are transformations of the natural orbitals. They are singly occupied and atom centered. Sulfur 3 p 2 MO; Occ=2. 0 Includes multi-reference character. It provides insight into the rearrangement of electrons during bonding. GVBL; Occ=1. 0 GVBR; Occ=1. 0

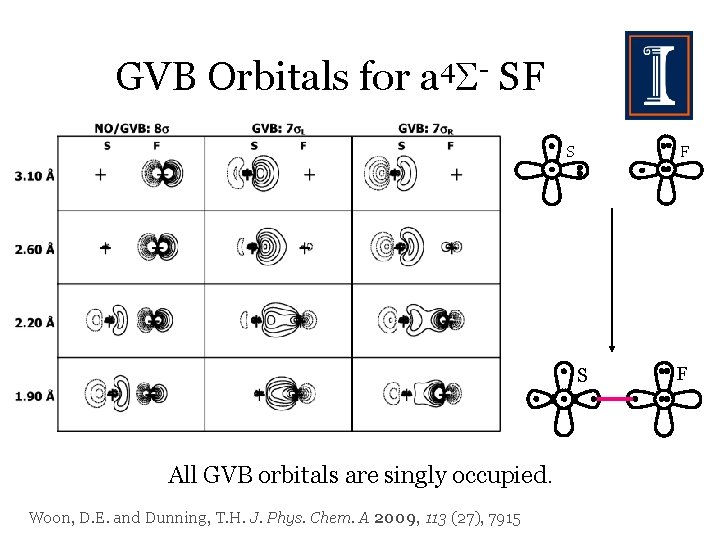
GVB Orbitals for a 4 - SF S All GVB orbitals are singly occupied. Woon, D. E. and Dunning, T. H. J. Phys. Chem. A 2009, 113 (27), 7915 F

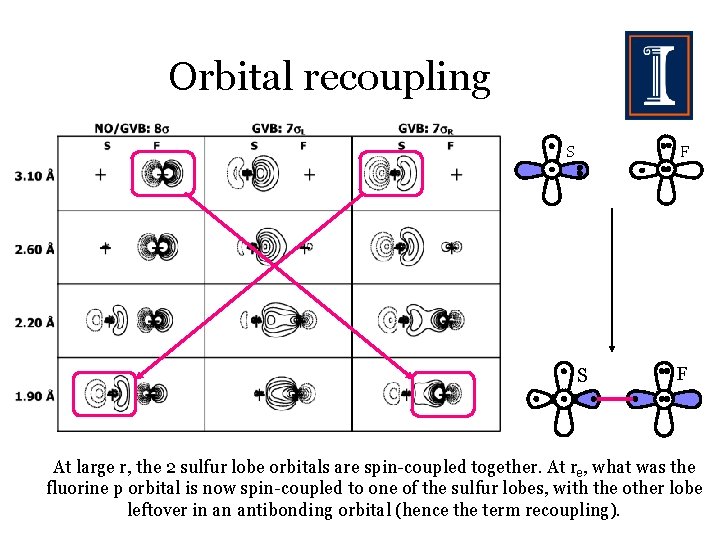
Orbital recoupling S F At large r, the 2 sulfur lobe orbitals are spin-coupled together. At re, what was the fluorine p orbital is now spin-coupled to one of the sulfur lobes, with the other lobe leftover in an antibonding orbital (hence the term recoupling).

Connection between RPB and hypervalency S F

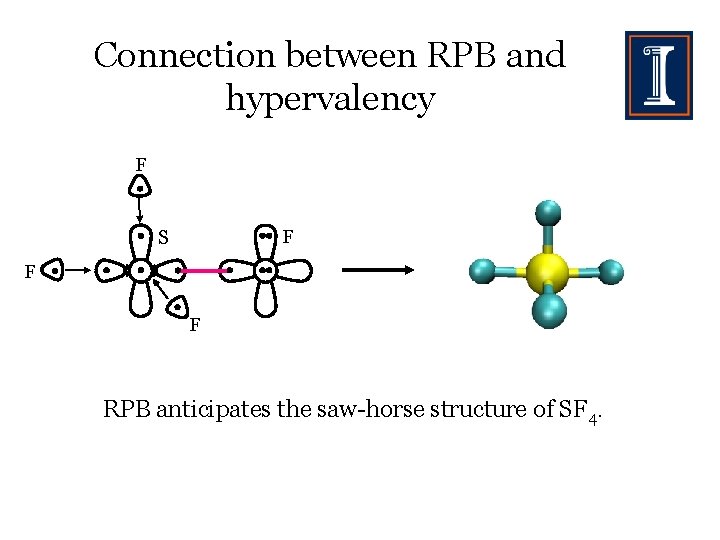
Connection between RPB and hypervalency F F S F F RPB anticipates the saw-horse structure of SF 4.

How do ligand properties affect recoupled pair bonds? • What happens if we replace the ligand (F) with a different ligand? F Cl, Br, I F OH, NH 2, CH 3 • What features determine the strength of a RPB? • Can we connect ligand properties to RPB strengths?

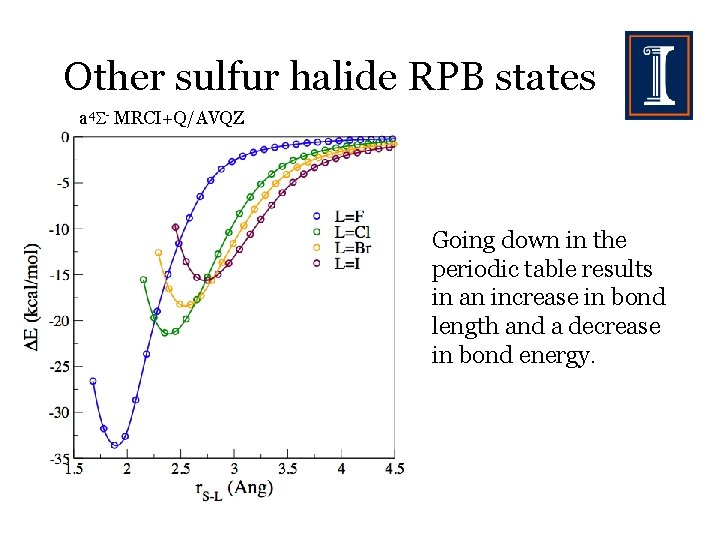
Other sulfur halide RPB states a 4 - MRCI+Q/AVQZ Going down in the periodic table results in an increase in bond length and a decrease in bond energy.

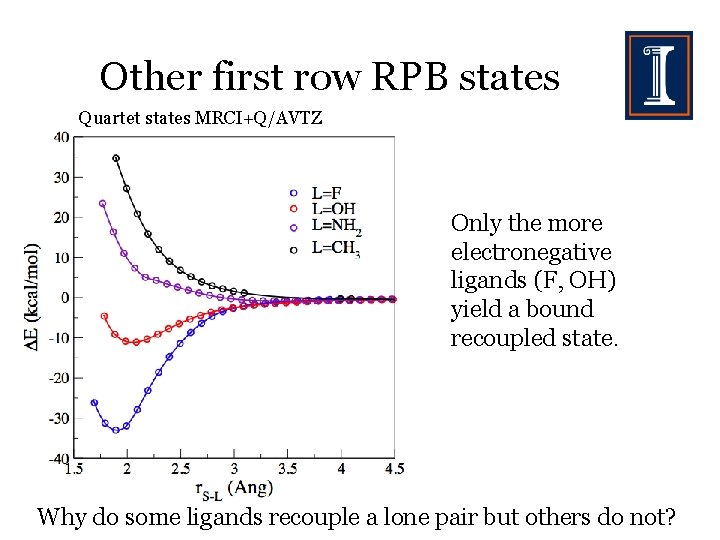
Other first row RPB states Quartet states MRCI+Q/AVTZ Only the more electronegative ligands (F, OH) yield a bound recoupled state. Why do some ligands recouple a lone pair but others do not?

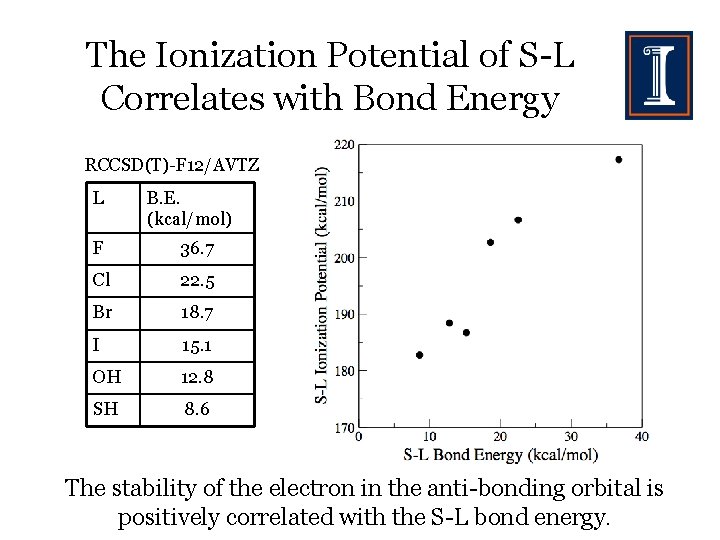
The Ionization Potential of S-L Correlates with Bond Energy RCCSD(T)-F 12/AVTZ L B. E. (kcal/mol) F 36. 7 Cl 22. 5 Br 18. 7 I 15. 1 OH 12. 8 SH 8. 6 The stability of the electron in the anti-bonding orbital is positively correlated with the S-L bond energy.

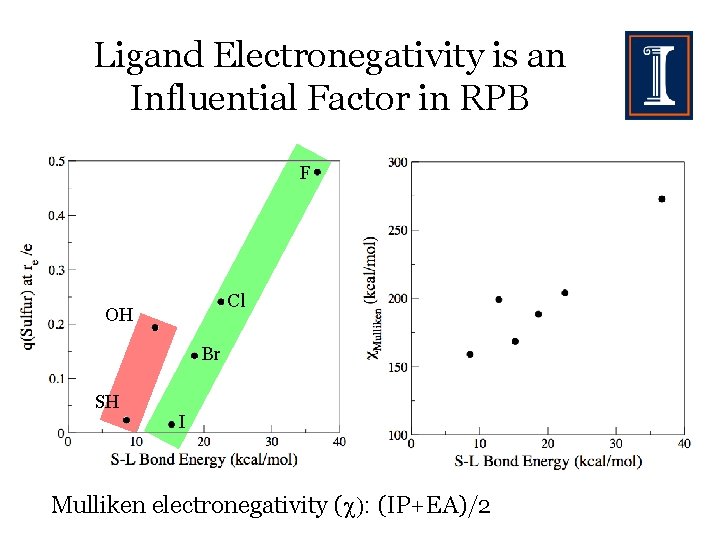
Ligand Electronegativity is an Influential Factor in RPB F Cl OH Br SH I Mulliken electronegativity ( : (IP+EA)/2

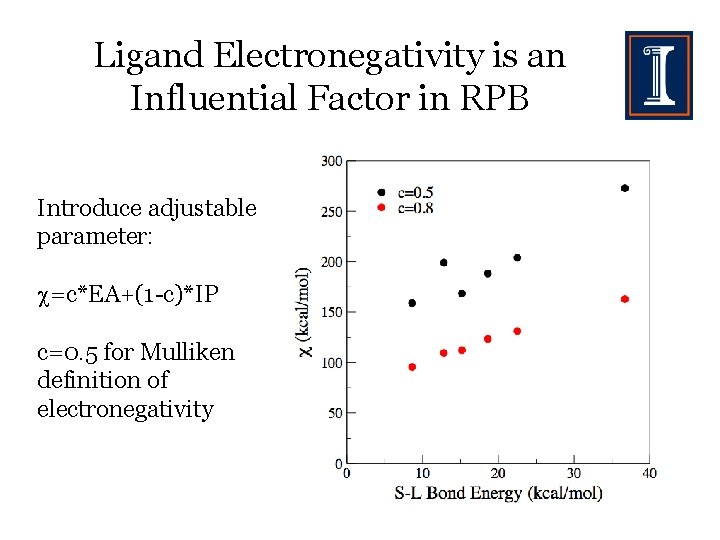
Ligand Electronegativity is an Influential Factor in RPB Introduce adjustable parameter: =c*EA+(1 -c)*IP c=0. 5 for Mulliken definition of electronegativity

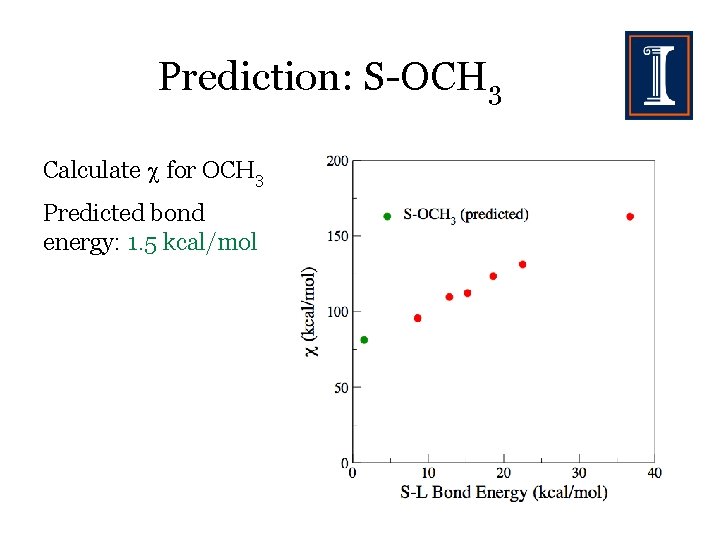
Prediction: S-OCH 3 Calculate for OCH 3 Predicted bond energy: 1. 5 kcal/mol

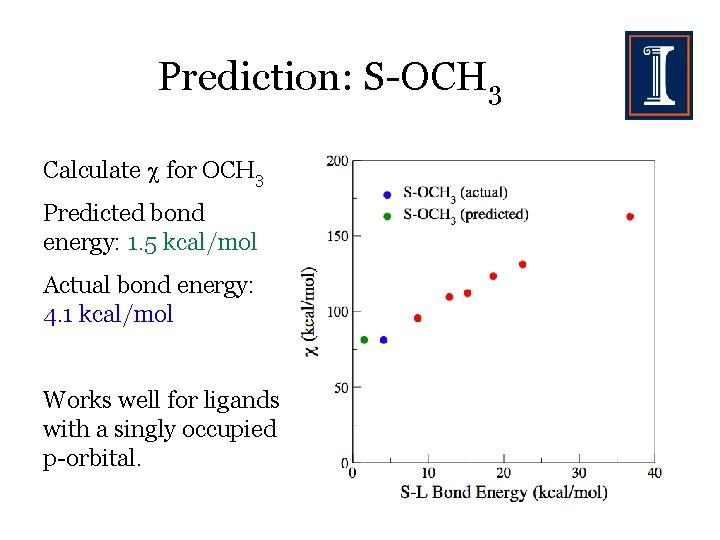
Prediction: S-OCH 3 Calculate for OCH 3 Predicted bond energy: 1. 5 kcal/mol Actual bond energy: 4. 1 kcal/mol Works well for ligands with a singly occupied p-orbital.

Conclusions • Recoupled pair bonding can explain hypervalency in the late p-block. • Ligand properties correlate well with recoupled pair bond strengths, providing the potential to quantitatively predict RPB strengths. • Electron affinity seems to play a particularly important role in determining the strength of a RPB.

Acknowledgements The Dunning Group