How has the periodic table developed over time

How has the periodic table developed over time? All pupils will be able to (Baseline): Describe how elements in the periodic table are organised by their atomic number and reactivity. Most pupils will be able to (Further): Explain how the position of an element in the periodic table is related to its atomic number and some of the steps in the development of the periodic table. Some pupils will be able to (Challenge): Predict possible reactions and probable reactivity of elements from their positions in the periodic table.
�http: //www. rsc. org/periodic-table or Dynamic Periodic Table (Interactive periodic tables) The Periodic Table of Videos �http: //www. bbc. co. uk/education/clips/z 4 snvcw (2 min explanation of periodic table Lower) �Mendeleev atomic number (2: 40 min Higher) �History of the periodic table (5 min long)

The Periodic Table – A Bit of History �The Great Chemist, Great Beard. modern periodic table was laid out by a man called Dimitri Mendeleev. �The periodic table is essentially a map of all of the known elements in the universe. �It does not look much at first glance, but is THE most important reference point for discovering what the universe

Greek philosophers 4 elements Aristotle, etc Earth Air Water Fire
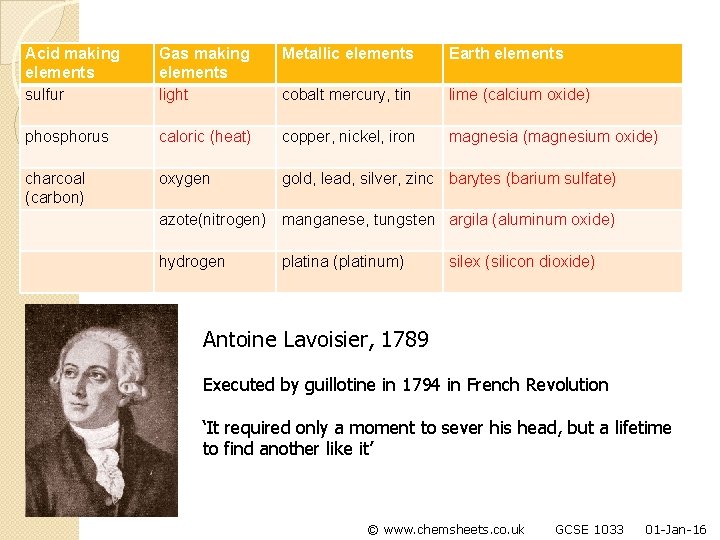
Acid making elements sulfur Gas making elements light Metallic elements Earth elements cobalt mercury, tin lime (calcium oxide) phosphorus caloric (heat) copper, nickel, iron magnesia (magnesium oxide) charcoal (carbon) oxygen gold, lead, silver, zinc barytes (barium sulfate) azote(nitrogen) manganese, tungsten argila (aluminum oxide) hydrogen platina (platinum) silex (silicon dioxide) Antoine Lavoisier, 1789 Executed by guillotine in 1794 in French Revolution ‘It required only a moment to sever his head, but a lifetime to find another like it’ © www. chemsheets. co. uk GCSE 1033 01 -Jan-16

Li 7 Ca 40 Cl 35. 5 Na 23 Sr 88 Br 80 K 39 Ba 137 I 127 7 + 39 2 40 + 137 2 35. 5 + 127 2 = 23 = 88. 5 = 81. 3 Johann Wolfgang Dobereiner, 1829
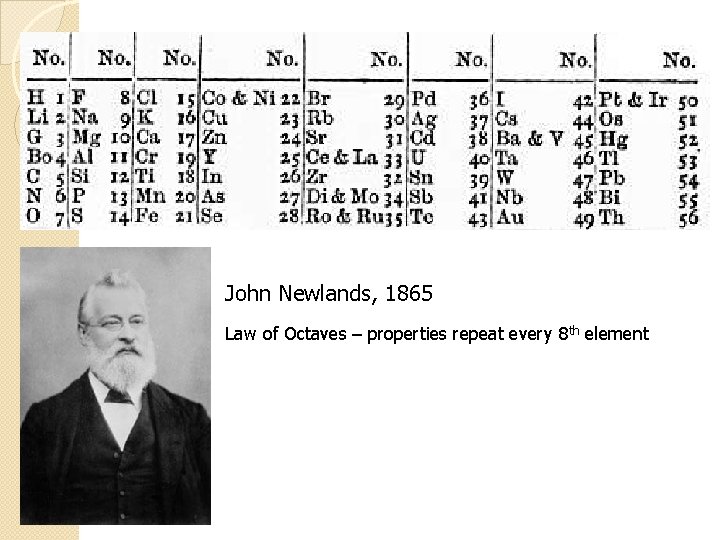
John Newlands, 1865 Law of Octaves – properties repeat every 8 th element
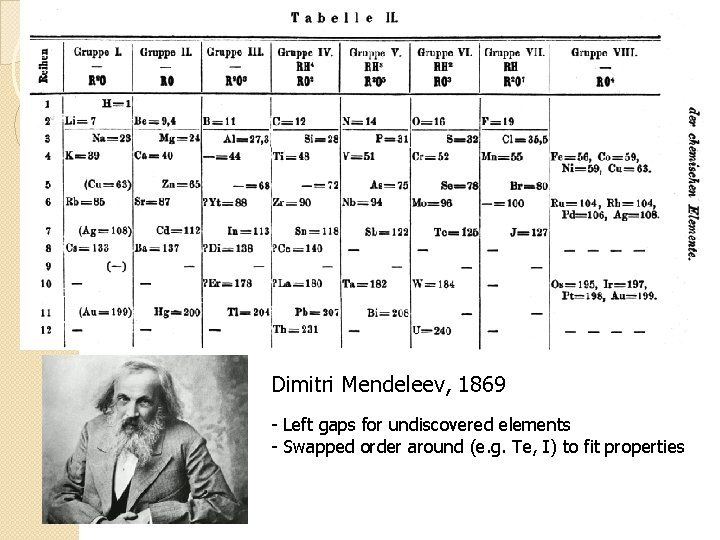
Dimitri Mendeleev, 1869 - Left gaps for undiscovered elements - Swapped order around (e. g. Te, I) to fit properties

�Suggest how Mendeleev may have been able to predict the properties of Rubidium?

Plenary �Interactive periodic table game (they will need to know the basic properties of metals and non-metals)
- Slides: 10