HOW ENZYMES WORK ENZYMES SPEED UP CHEMICAL REACTIONS

![Michaelis-Menten kinetics V 0 varies with [S] Vmax approached asymptotically V 0 is moles Michaelis-Menten kinetics V 0 varies with [S] Vmax approached asymptotically V 0 is moles](https://slidetodoc.com/presentation_image_h2/4885ab2162a46a668cd6ce6ea5a3e5a7/image-16.jpg)
![Determining initial velocity (when [P] is low) Determining initial velocity (when [P] is low)](https://slidetodoc.com/presentation_image_h2/4885ab2162a46a668cd6ce6ea5a3e5a7/image-17.jpg)
![Steady-state & pre-steady-state conditions At equilibrium, no net change of [S] & [P] or Steady-state & pre-steady-state conditions At equilibrium, no net change of [S] & [P] or](https://slidetodoc.com/presentation_image_h2/4885ab2162a46a668cd6ce6ea5a3e5a7/image-18.jpg)
![Range of Km values Km provides approximation of [S] in vivo for many enzymes Range of Km values Km provides approximation of [S] in vivo for many enzymes](https://slidetodoc.com/presentation_image_h2/4885ab2162a46a668cd6ce6ea5a3e5a7/image-20.jpg)
- Slides: 29

HOW ENZYMES WORK

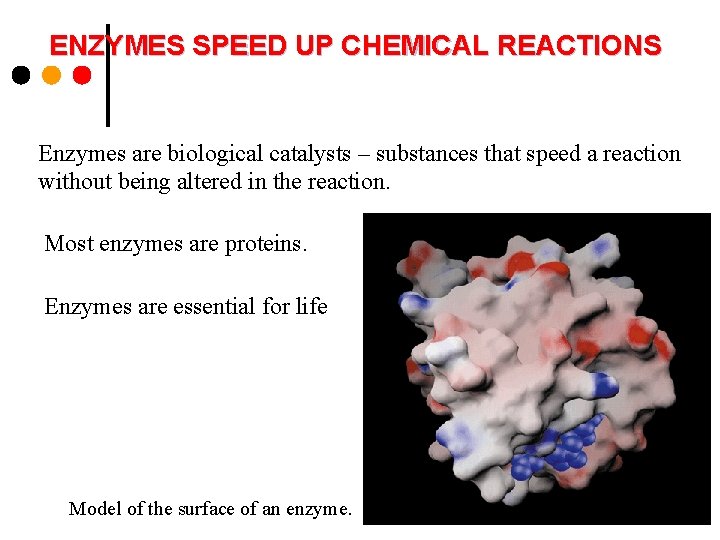
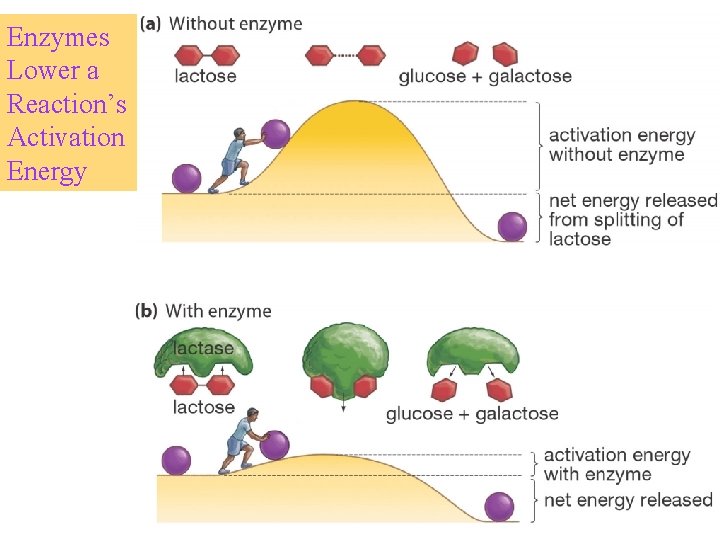
ENZYMES SPEED UP CHEMICAL REACTIONS Enzymes are biological catalysts – substances that speed a reaction without being altered in the reaction. Most enzymes are proteins. Enzymes are essential for life. Model of the surface of an enzyme.

v Enzymes �� Cofactors �� Coenzymes �� Holoenzyme �� Apoenzyme

How Enzymes Work? • Body conditions(temperature, pressure etc. ) not good for reaction • Only enzymes can catalyse the reactions in this conditions • A special environment inside enzymes for reaction ACTIVE SITE • Molecule binds active site SUBSTRATE




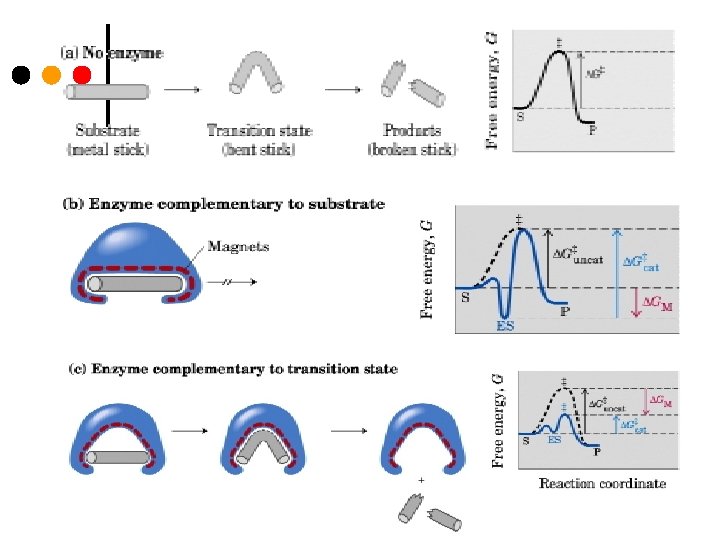
Enzymes Lower a Reaction’s Activation Energy

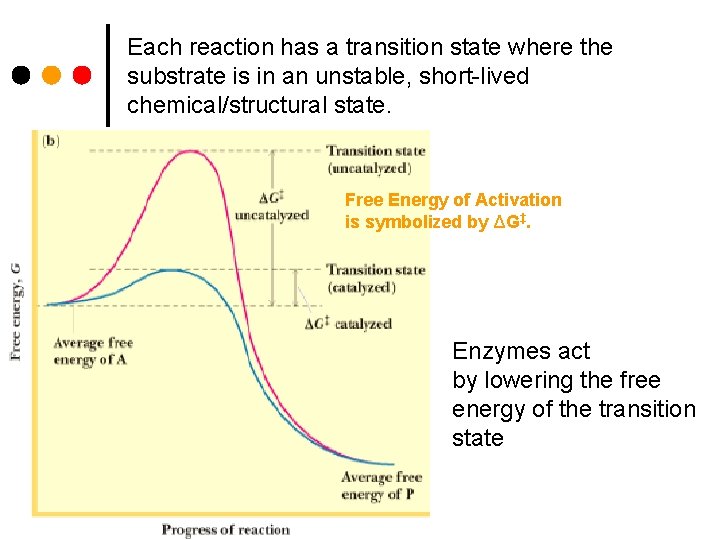
Each reaction has a transition state where the substrate is in an unstable, short-lived chemical/structural state. Free Energy of Activation is symbolized by ΔG‡. Enzymes act by lowering the free energy of the transition state

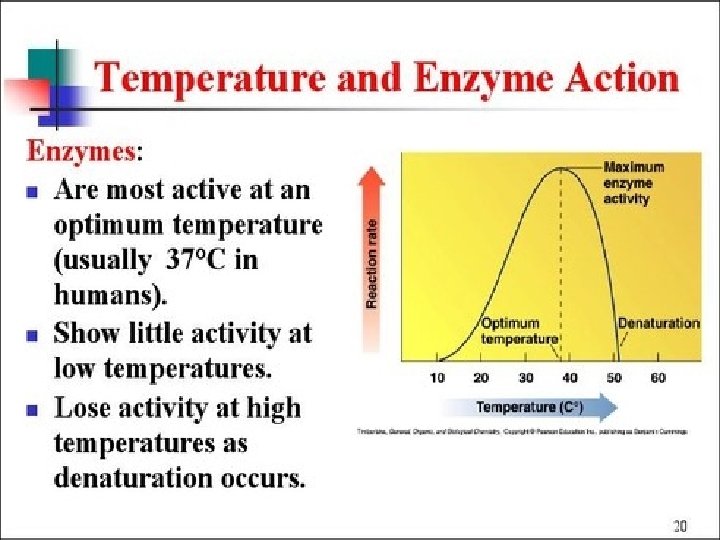
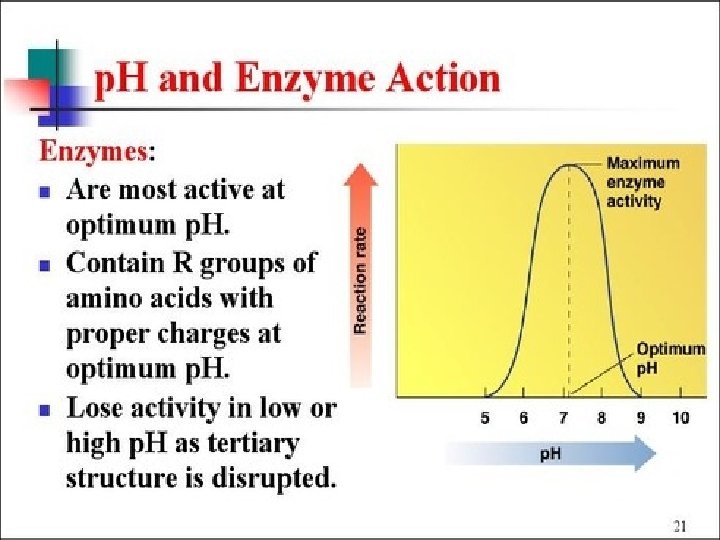
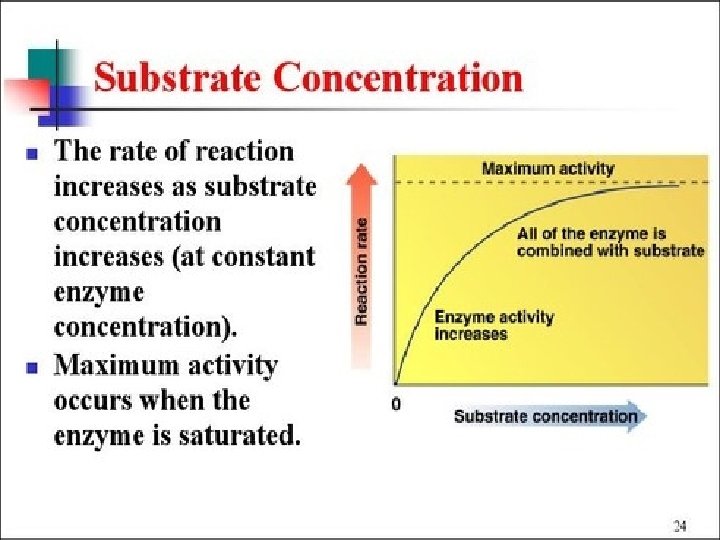
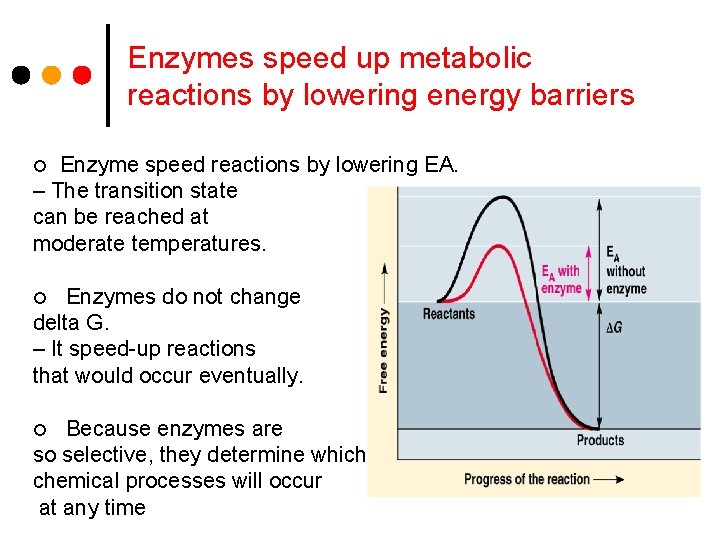
Enzymes speed up metabolic reactions by lowering energy barriers Enzyme speed reactions by lowering EA. – The transition state can be reached at moderate temperatures. ¢ Enzymes do not change delta G. – It speed-up reactions that would occur eventually. ¢ Because enzymes are so selective, they determine which chemical processes will occur at any time ¢

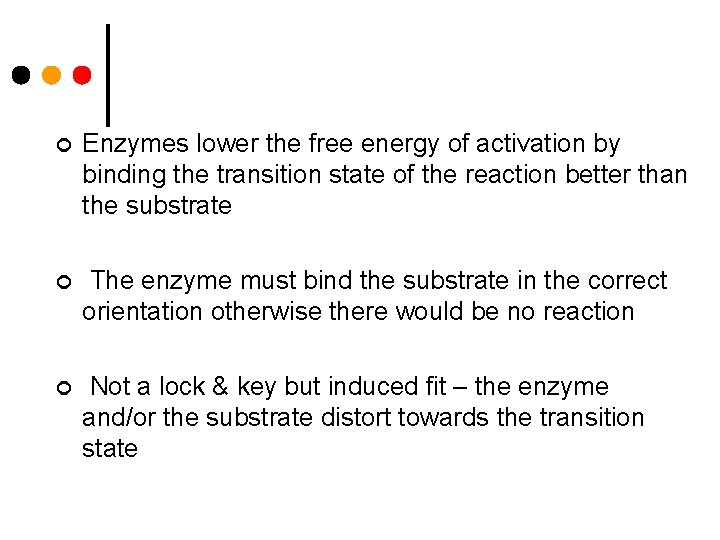
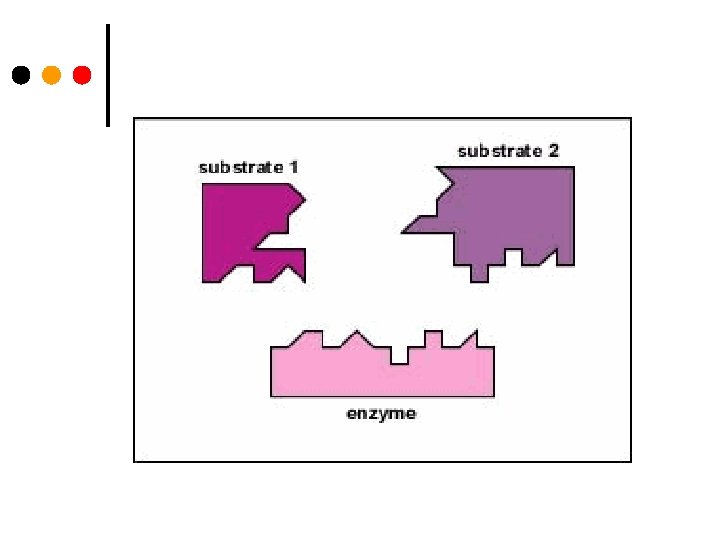
¢ Enzymes lower the free energy of activation by binding the transition state of the reaction better than the substrate ¢ The enzyme must bind the substrate in the correct orientation otherwise there would be no reaction ¢ Not a lock & key but induced fit – the enzyme and/or the substrate distort towards the transition state


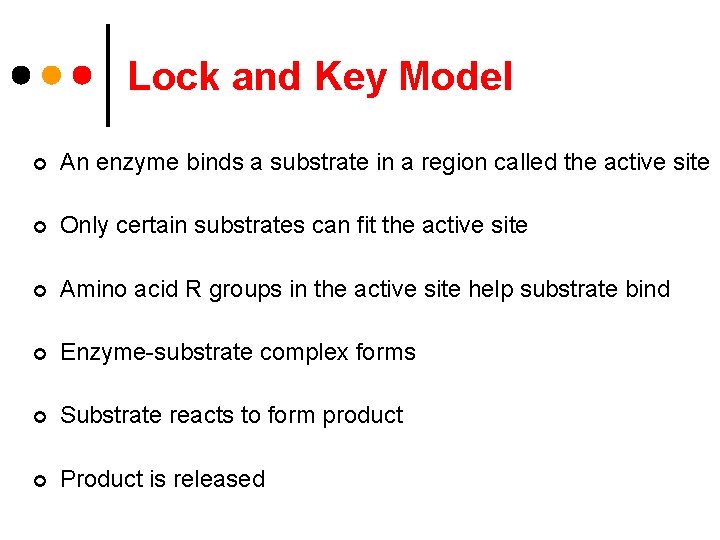
Lock and Key Model ¢ An enzyme binds a substrate in a region called the active site ¢ Only certain substrates can fit the active site ¢ Amino acid R groups in the active site help substrate bind ¢ Enzyme-substrate complex forms ¢ Substrate reacts to form product ¢ Product is released


Enzyme Kinetics - Kinetics The study of the rate of change. - Enzyme Kinetics Rate of chemical reactions mediated by enzymes. Enzymes can increase reaction rate by favoring or enabling a different reaction pathway with a lower activation energy, making it easier for the reaction to occur.
![MichaelisMenten kinetics V 0 varies with S Vmax approached asymptotically V 0 is moles Michaelis-Menten kinetics V 0 varies with [S] Vmax approached asymptotically V 0 is moles](https://slidetodoc.com/presentation_image_h2/4885ab2162a46a668cd6ce6ea5a3e5a7/image-16.jpg)
Michaelis-Menten kinetics V 0 varies with [S] Vmax approached asymptotically V 0 is moles of product formed per sec. when [P] is low (close to zero time) E + S ES E + P Michaelis-Menten Model V 0 = Vmax x[S]/([S] + Km) Michaelis-Menten Equation
![Determining initial velocity when P is low Determining initial velocity (when [P] is low)](https://slidetodoc.com/presentation_image_h2/4885ab2162a46a668cd6ce6ea5a3e5a7/image-17.jpg)
Determining initial velocity (when [P] is low)
![Steadystate presteadystate conditions At equilibrium no net change of S P or Steady-state & pre-steady-state conditions At equilibrium, no net change of [S] & [P] or](https://slidetodoc.com/presentation_image_h2/4885ab2162a46a668cd6ce6ea5a3e5a7/image-18.jpg)
Steady-state & pre-steady-state conditions At equilibrium, no net change of [S] & [P] or of [ES] & [E] At pre-steady-state, [P] is low (close to zero time), hence, V 0 for initial reaction velocity At pre-steady state, we can ignore the back reactions

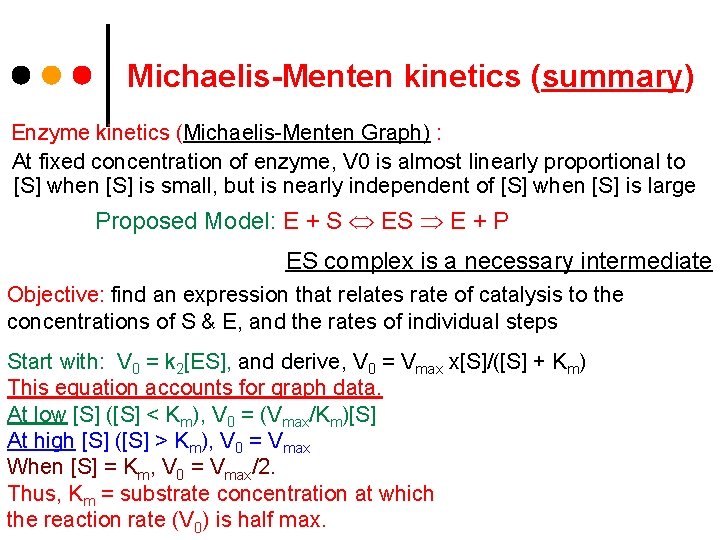
Michaelis-Menten kinetics (summary) Enzyme kinetics (Michaelis-Menten Graph) : At fixed concentration of enzyme, V 0 is almost linearly proportional to [S] when [S] is small, but is nearly independent of [S] when [S] is large Proposed Model: E + S E + P ES complex is a necessary intermediate Objective: find an expression that relates rate of catalysis to the concentrations of S & E, and the rates of individual steps Start with: V 0 = k 2[ES], and derive, V 0 = Vmax x[S]/([S] + Km) This equation accounts for graph data. At low [S] ([S] < Km), V 0 = (Vmax/Km)[S] At high [S] ([S] > Km), V 0 = Vmax When [S] = Km, V 0 = Vmax/2. Thus, Km = substrate concentration at which the reaction rate (V 0) is half max.
![Range of Km values Km provides approximation of S in vivo for many enzymes Range of Km values Km provides approximation of [S] in vivo for many enzymes](https://slidetodoc.com/presentation_image_h2/4885ab2162a46a668cd6ce6ea5a3e5a7/image-20.jpg)
Range of Km values Km provides approximation of [S] in vivo for many enzymes

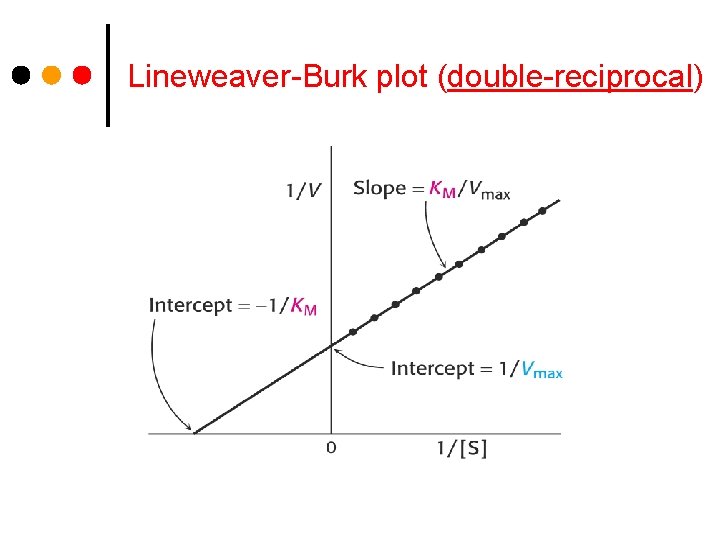
Lineweaver-Burk plot (double-reciprocal)

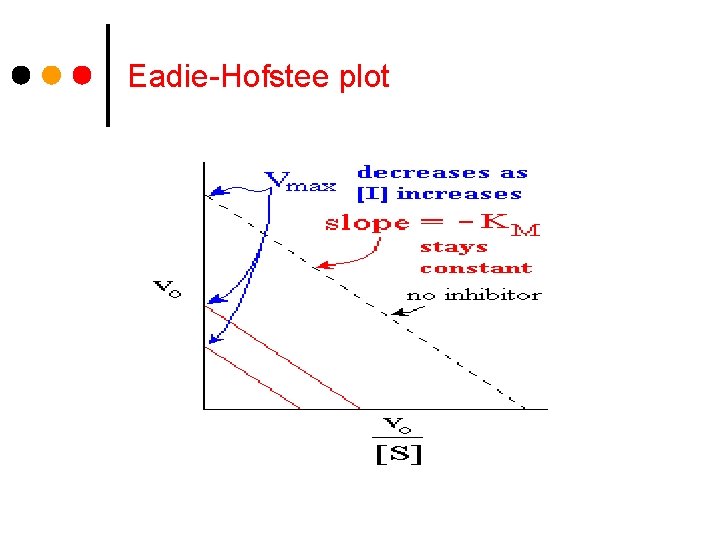
Eadie-Hofstee plot

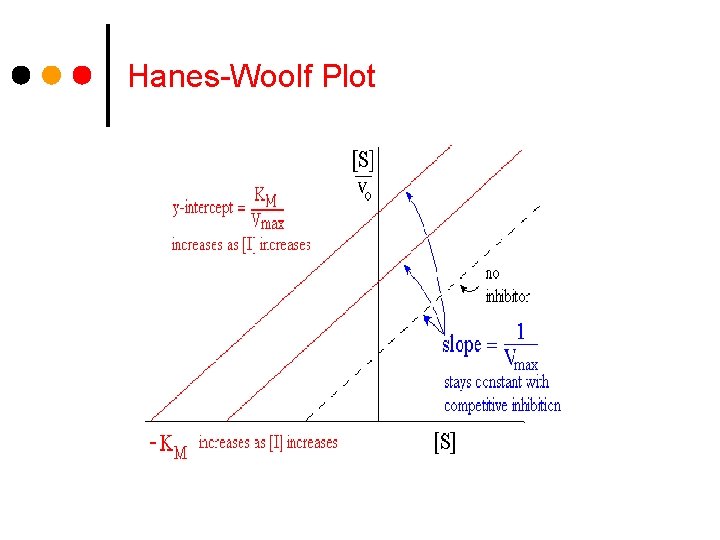
Hanes-Woolf Plot