How enzymes catalyze reactions Organic chemistry of vitamins












- Slides: 41

How enzymes catalyze reactions? Organic chemistry of vitamins

�Book: Bruice �Chapter: 17 �Pages: 506 -524

Enzyme-catalyzed reactions �Reactant of an enzyme catalysis – substrate �Substrate + Enzyme -> Product �Substrate binds in a pocket of enzyme – active site �All bond-breaking and bon-forming steps occur in that moment �Enzymes – specific for the substrates �Specificity of enzymes – molecular recognition

�Specificity – results from its conformation and the particular amino acid side chains (α-substituents) that are at the active site �Example: AA with negatively charged side chain associates with a positively charged group on the substrate

Factors �Influence catalytic ability of enzymes: Ø • Reacting groups are brought together at the active site in the proper orientation for reaction. Ø • Some of the amino acid side chains of the enzyme serve as catalysts. These are positioned relative to the substrate precisely where they are needed for catalysis. Ø • Amino acid side chains can stabilize transition states and intermediates—by van der Waals interactions, electrostatic interactions, and hydrogen bonding—which makes them easier to form.

Enzyme mechanism �“ase” – tells something about the reaction it catalyzes �Example: glucose-6 -phosphate isomerase �catalyzes an isomerization reaction that converts glucose-6 -phosphate to fructose-6 -phosphate �open-chain form of glucose is an aldohexose �open-chain form of fructose is a ketohexose �glucose-6 -phosphate isomerase—converts an aldose to a ketose

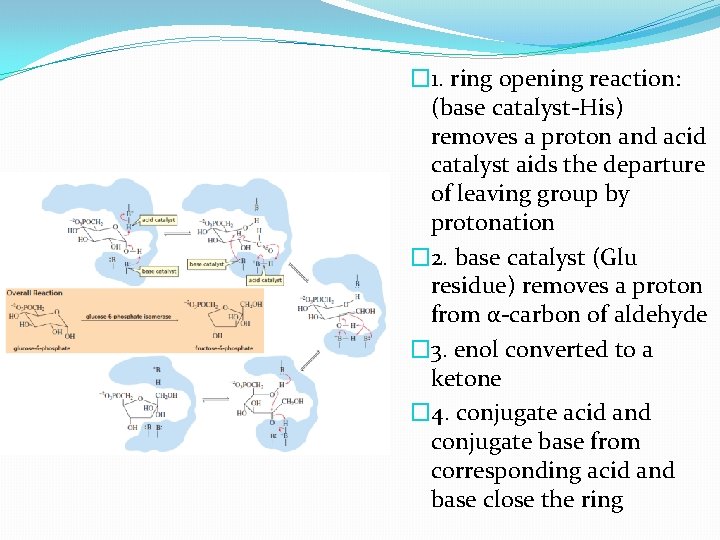
�Enzyme must open the six-membered-ring sugar �convert it to the five-membered-ring sugar �This enzyme – has at least 3 catalytic groups at its active site �One functioning as an acid catalyst and two acting as base catalyst

� 1. ring opening reaction: (base catalyst-His) removes a proton and acid catalyst aids the departure of leaving group by protonation � 2. base catalyst (Glu residue) removes a proton from α-carbon of aldehyde � 3. enol converted to a ketone � 4. conjugate acid and conjugate base from corresponding acid and base close the ring

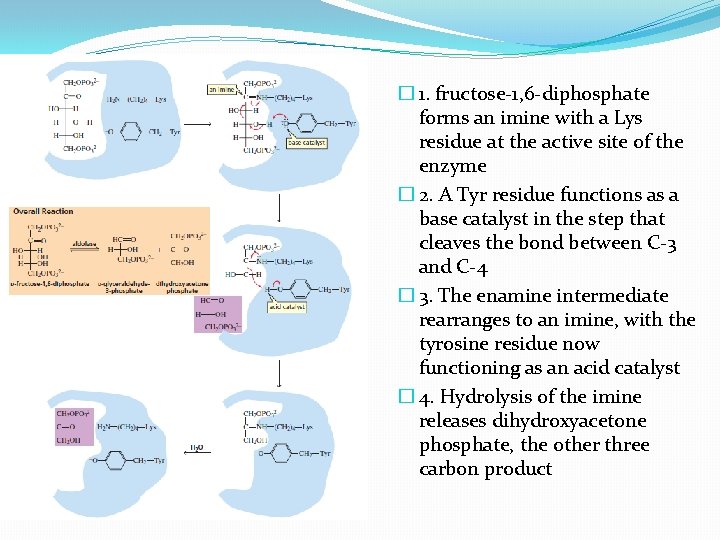
The mechanism of aldolase �Involved in glycolysis �D-glucose is substrate �Final product – 2 molecules of pyruvate �six-carbon compound must be cleaved into two threecarbon compounds �Aldolase - catalyzes this cleavage �The enzyme is called aldolase because the reverse reaction is an aldol addition

� 1. fructose-1, 6 -diphosphate forms an imine with a Lys residue at the active site of the enzyme � 2. A Tyr residue functions as a base catalyst in the step that cleaves the bond between C-3 and C-4 � 3. The enamine intermediate rearranges to an imine, with the tyrosine residue now functioning as an acid catalyst � 4. Hydrolysis of the imine releases dihydroxyacetone phosphate, the other three carbon product

Coenzymes and vitamins �Coenzymes – enzyme helpers �organic molecules �assist enzymes in catalyzing certain reactions that cannot be catalyzed by the amino acid side chains of the enzyme alone �derived from organic compounds commonly known as vitamins �Vitamin - substance needed in small amounts for normal body function that the body cannot synthesize �The body synthesizes the coenzyme from the vitamin

� 2 classes: water and fat soluble �Fat soluble: A, D, E and K Ø Vitamin K - only water-insoluble vitamin currently known to be a precursor for a coenzyme Ø Vitamin A - for proper vision Ø Vitamin D - regulates calcium and phosphate metabolism Ø Vitamin E – antioxidant �Water soluble: B complex (precursors for coenzymes) and vitamin C (no precursor) Ø vitamin C is a radical inhibitor - must be included in their diets


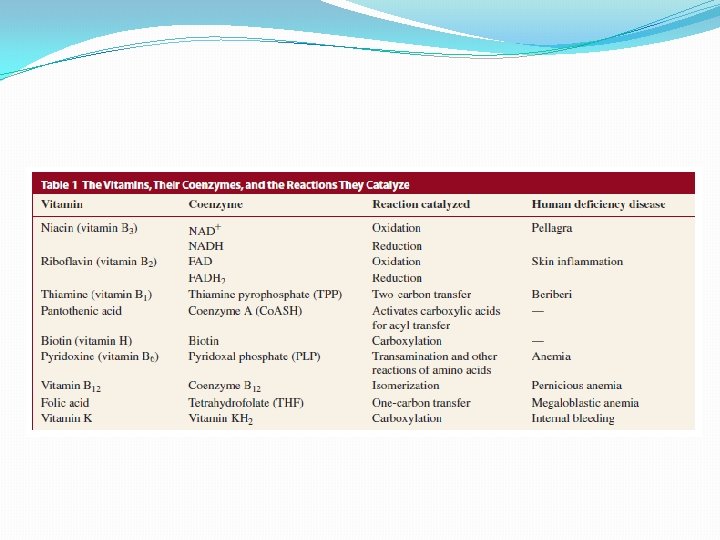
Niacin �For redox reactions �Needs coenzyme – AA cannot perform redox reactions �coenzyme serves as the oxidizing or reducing agent �enzyme’s role is to hold the substrate and coenzyme together so that the oxidation or reduction reaction can take place �most commonly used: nicotinamide adenine dinucleotide (NAD+)

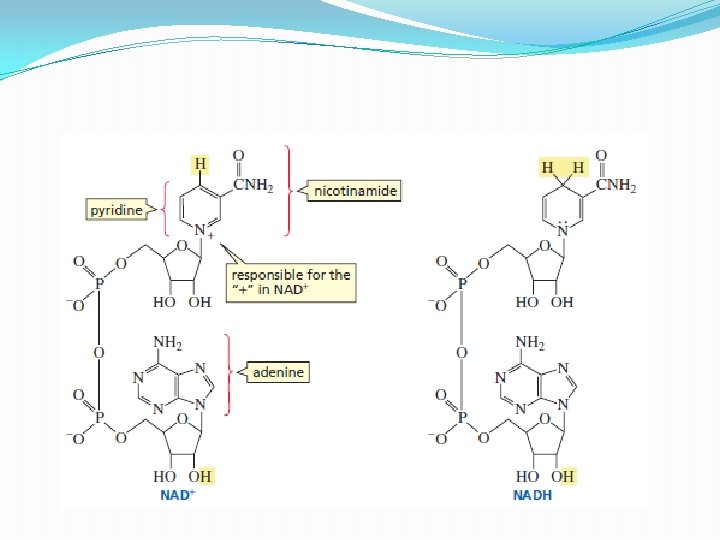
�composed of two nucleotides �linked together through their phosphate groups �Nucleotide - heterocyclic compound attached to C-1 of a phosphorylated ribose �Heterocyclic compound – one or more of the ring atoms is an atom other than carbon �The heterocyclic component of one of the nucleotides of NAD+ is nicotinamide, and the heterocyclic component of the other is adenine �The positive charge in the NAD+ abbreviation indicates the positively charged nitrogen of the substituted pyridine ring


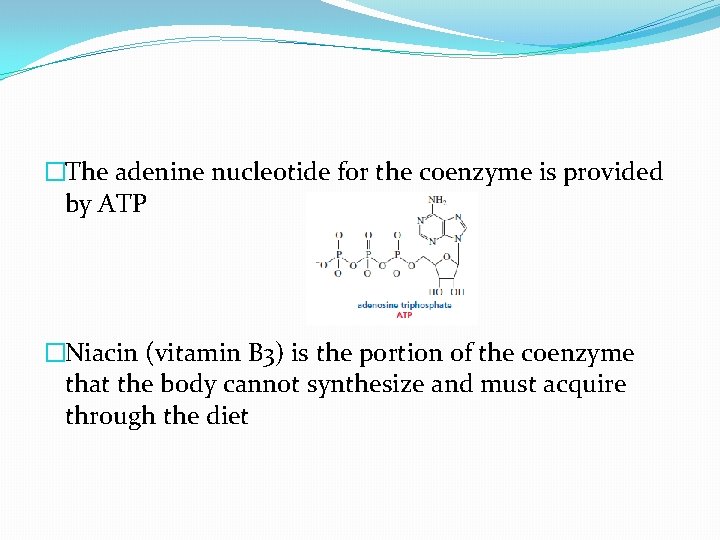
�The adenine nucleotide for the coenzyme is provided by ATP �Niacin (vitamin B 3) is the portion of the coenzyme that the body cannot synthesize and must acquire through the diet

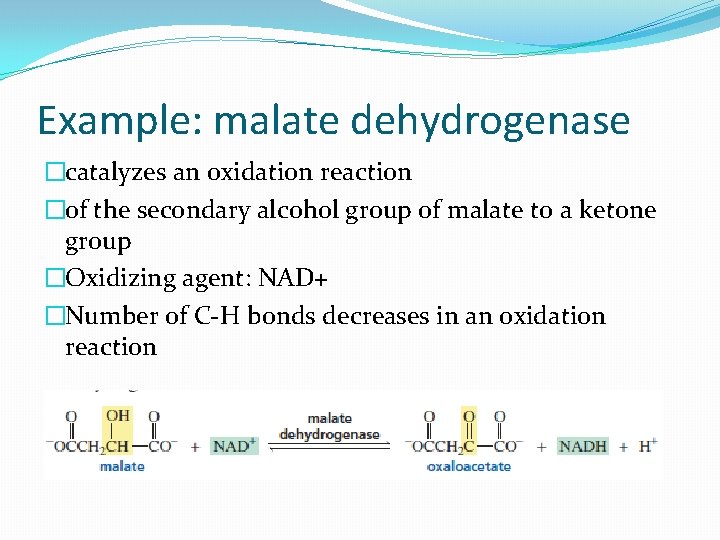
Example: malate dehydrogenase �catalyzes an oxidation reaction �of the secondary alcohol group of malate to a ketone group �Oxidizing agent: NAD+ �Number of C-H bonds decreases in an oxidation reaction

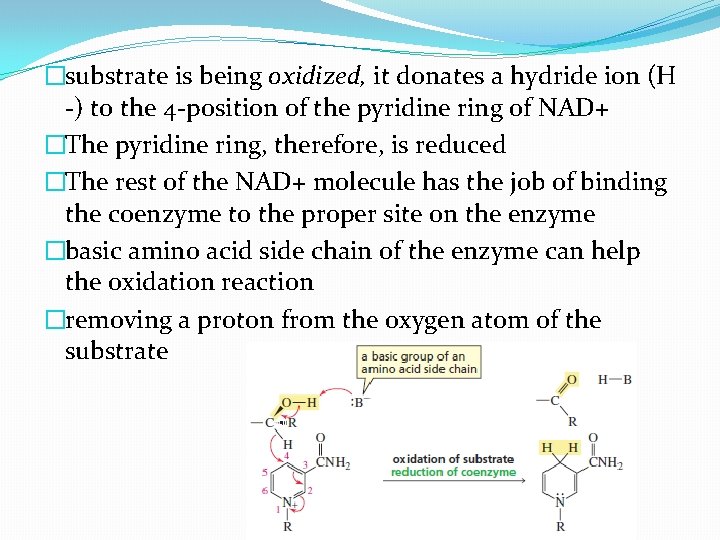
�substrate is being oxidized, it donates a hydride ion (H -) to the 4 -position of the pyridine ring of NAD+ �The pyridine ring, therefore, is reduced �The rest of the NAD+ molecule has the job of binding the coenzyme to the proper site on the enzyme �basic amino acid side chain of the enzyme can help the oxidation reaction �removing a proton from the oxygen atom of the substrate

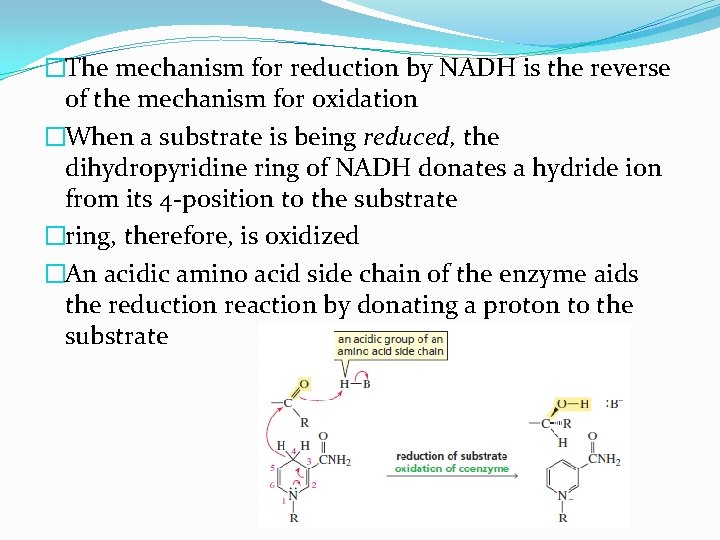
�The mechanism for reduction by NADH is the reverse of the mechanism for oxidation �When a substrate is being reduced, the dihydropyridine ring of NADH donates a hydride ion from its 4 -position to the substrate �ring, therefore, is oxidized �An acidic amino acid side chain of the enzyme aids the reduction reaction by donating a proton to the substrate

Niacin deficiency �Causes pellagra �Disease that begins with dermatitis and ultimately causes insanity and death �Reported in 1927 in USA (120, 000 cases) �among poor people with unvaried diets

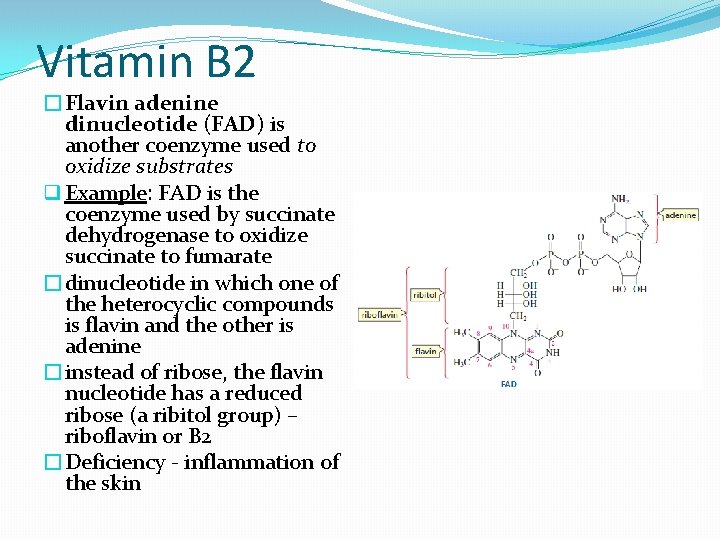
Vitamin B 2 �Flavin adenine dinucleotide (FAD) is another coenzyme used to oxidize substrates q Example: FAD is the coenzyme used by succinate dehydrogenase to oxidize succinate to fumarate �dinucleotide in which one of the heterocyclic compounds is flavin and the other is adenine �instead of ribose, the flavin nucleotide has a reduced ribose (a ribitol group) – riboflavin or B 2 �Deficiency - inflammation of the skin

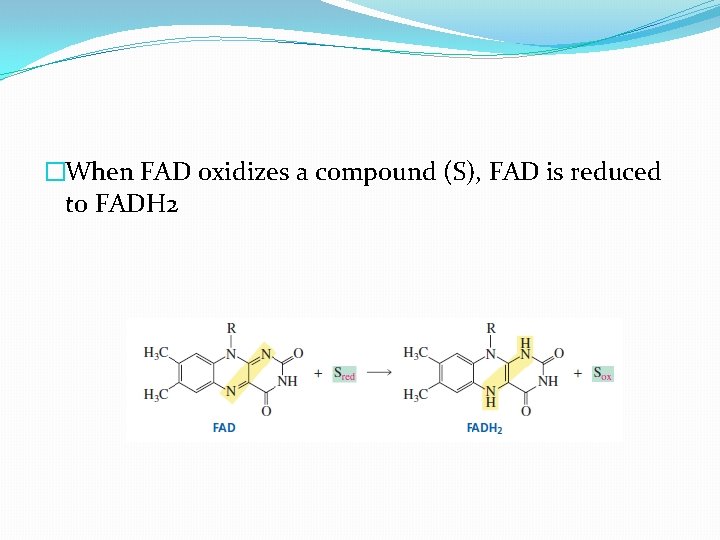
�When FAD oxidizes a compound (S), FAD is reduced to FADH 2

NAD+ or FAD? �NAD+ - in enzyme-catalyzed oxidation reactions involving carbonyl compounds �alcohols being oxidized to ketones, aldehydes, or carboxylic acids �FAD - coenzyme used in other types of oxidations

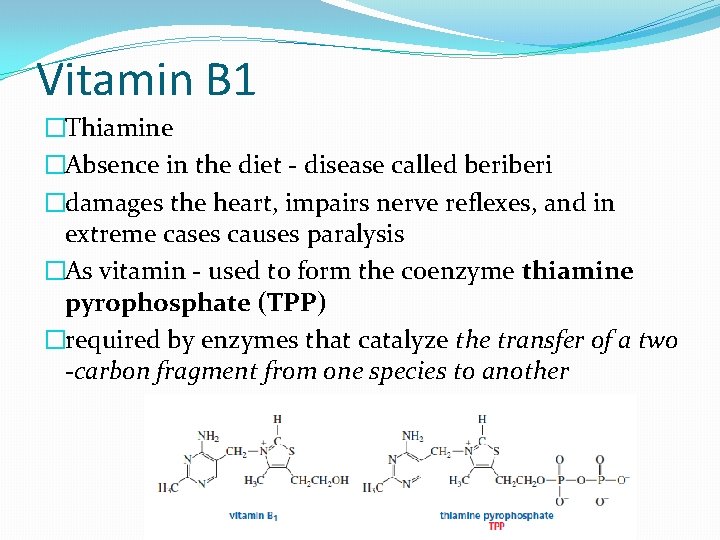
Vitamin B 1 �Thiamine �Absence in the diet - disease called beri �damages the heart, impairs nerve reflexes, and in extreme cases causes paralysis �As vitamin - used to form the coenzyme thiamine pyrophosphate (TPP) �required by enzymes that catalyze the transfer of a two -carbon fragment from one species to another

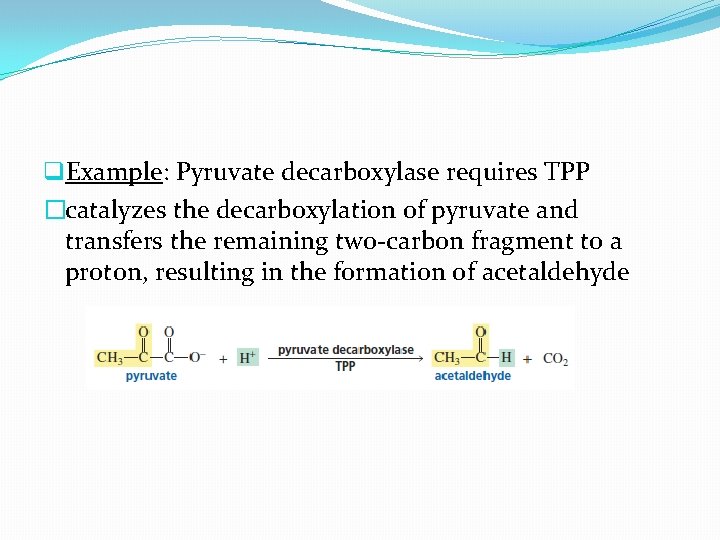
q. Example: Pyruvate decarboxylase requires TPP �catalyzes the decarboxylation of pyruvate and transfers the remaining two-carbon fragment to a proton, resulting in the formation of acetaldehyde

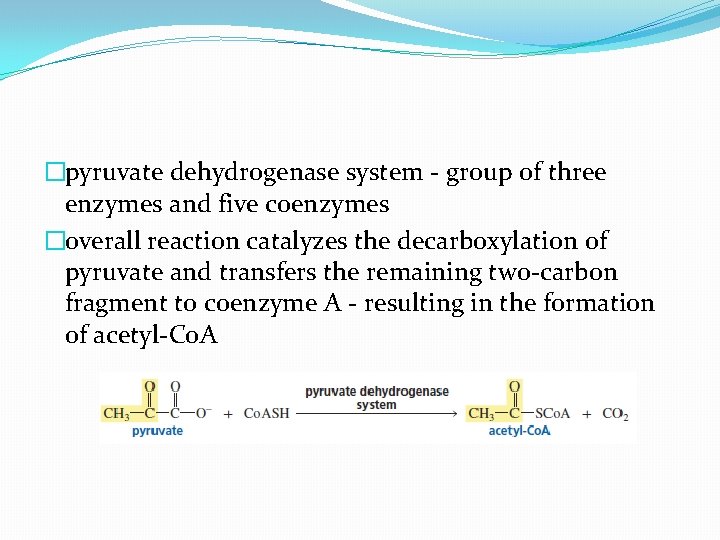
�pyruvate dehydrogenase system - group of three enzymes and five coenzymes �overall reaction catalyzes the decarboxylation of pyruvate and transfers the remaining two-carbon fragment to coenzyme A - resulting in the formation of acetyl-Co. A

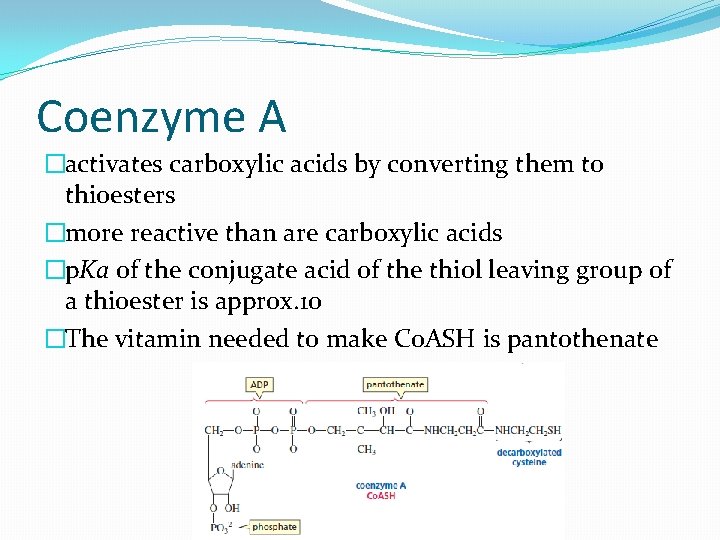
Coenzyme A �activates carboxylic acids by converting them to thioesters �more reactive than are carboxylic acids �p. Ka of the conjugate acid of the thiol leaving group of a thioester is approx. 10 �The vitamin needed to make Co. ASH is pantothenate

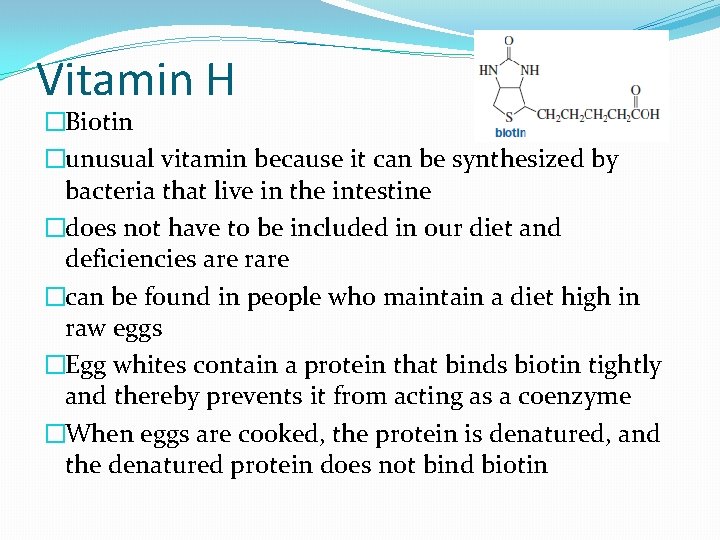
Vitamin H �Biotin �unusual vitamin because it can be synthesized by bacteria that live in the intestine �does not have to be included in our diet and deficiencies are rare �can be found in people who maintain a diet high in raw eggs �Egg whites contain a protein that binds biotin tightly and thereby prevents it from acting as a coenzyme �When eggs are cooked, the protein is denatured, and the denatured protein does not bind biotin

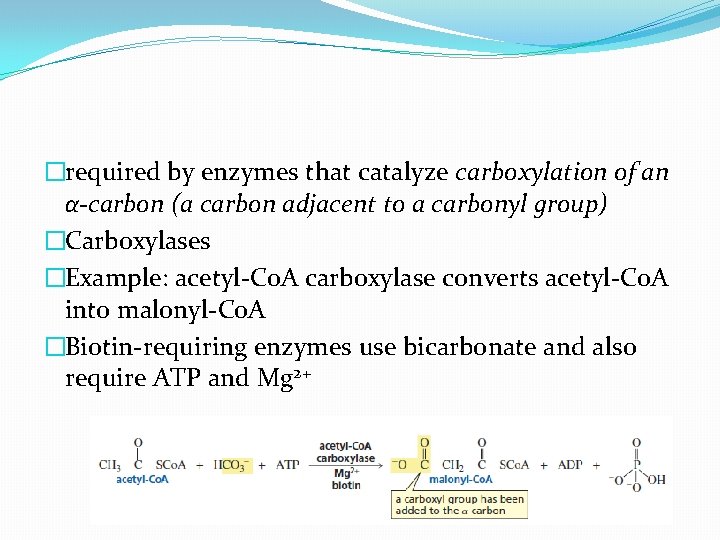
�required by enzymes that catalyze carboxylation of an α-carbon (a carbon adjacent to a carbonyl group) �Carboxylases �Example: acetyl-Co. A carboxylase converts acetyl-Co. A into malonyl-Co. A �Biotin-requiring enzymes use bicarbonate and also require ATP and Mg 2+

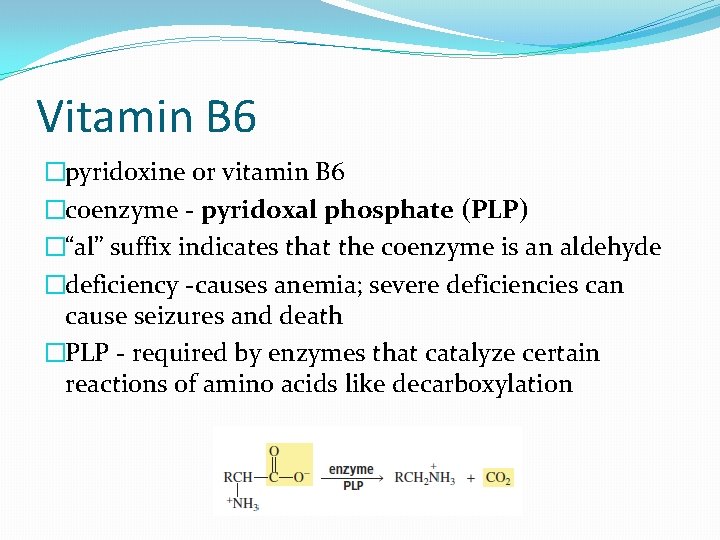
Vitamin B 6 �pyridoxine or vitamin B 6 �coenzyme - pyridoxal phosphate (PLP) �“al” suffix indicates that the coenzyme is an aldehyde �deficiency -causes anemia; severe deficiencies can cause seizures and death �PLP - required by enzymes that catalyze certain reactions of amino acids like decarboxylation

Vitamin B 12 �coenzyme B 12 �Has CN or HO- group coordinated with Cobalt �Humans must obtain all their vitamin B 12 from their diet, particularly from meat �deficiency - pernicious anemia �Most deficiencies are caused by the intestines’ inability to absorb the vitamin

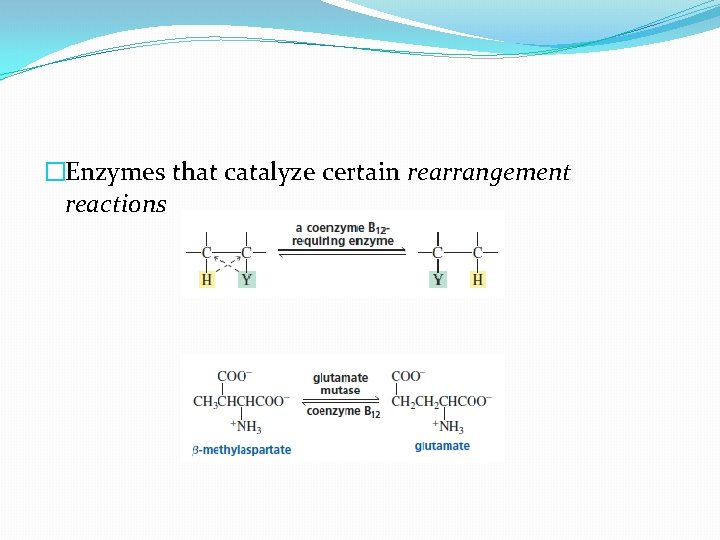
�Enzymes that catalyze certain rearrangement reactions

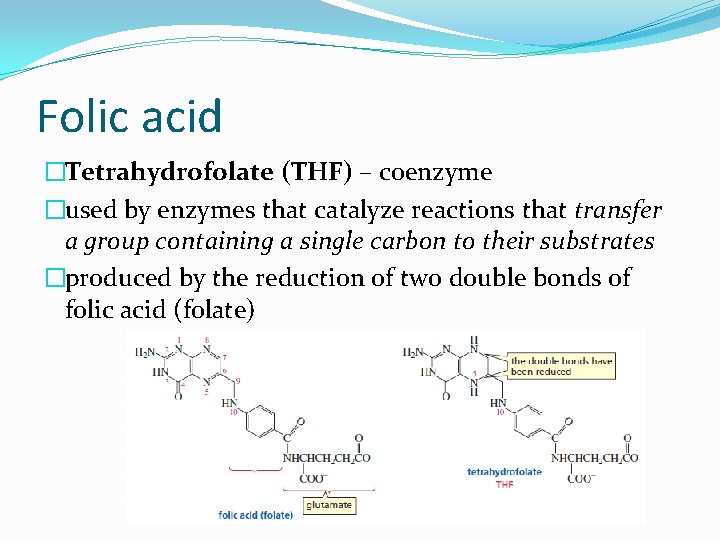
Folic acid �Tetrahydrofolate (THF) – coenzyme �used by enzymes that catalyze reactions that transfer a group containing a single carbon to their substrates �produced by the reduction of two double bonds of folic acid (folate)

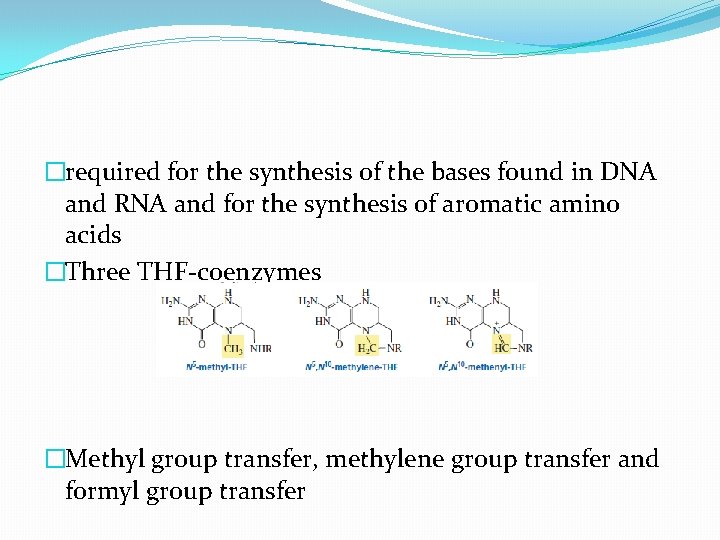
�required for the synthesis of the bases found in DNA and RNA and for the synthesis of aromatic amino acids �Three THF-coenzymes �Methyl group transfer, methylene group transfer and formyl group transfer

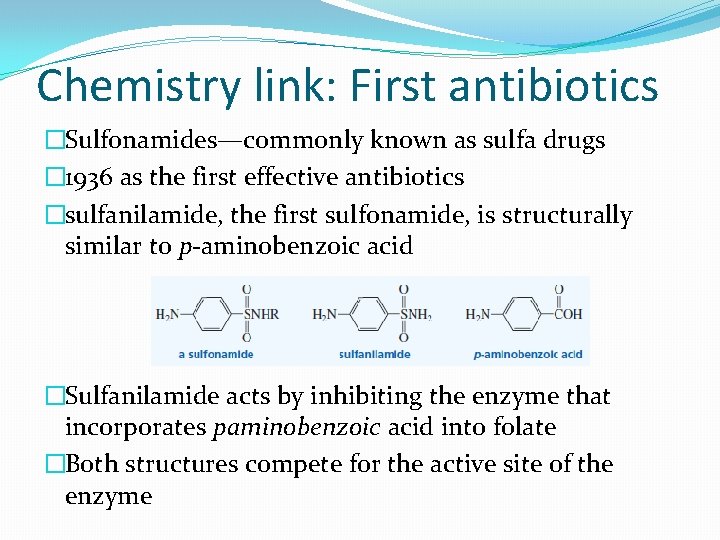
Chemistry link: First antibiotics �Sulfonamides—commonly known as sulfa drugs � 1936 as the first effective antibiotics �sulfanilamide, the first sulfonamide, is structurally similar to p-aminobenzoic acid �Sulfanilamide acts by inhibiting the enzyme that incorporates paminobenzoic acid into folate �Both structures compete for the active site of the enzyme

Vitamin K �for proper clotting of blood �K – koagulation (German word for clotting) �Process requires Ca 2+ �Vitamin K needed for proper Ca 2+ binding �found in the leaves of green plants �Deficiencies in the vitamin are rare because it is synthesized by intestinal bacteria �Vitamin KH 2 is the coenzyme form of the vitamin

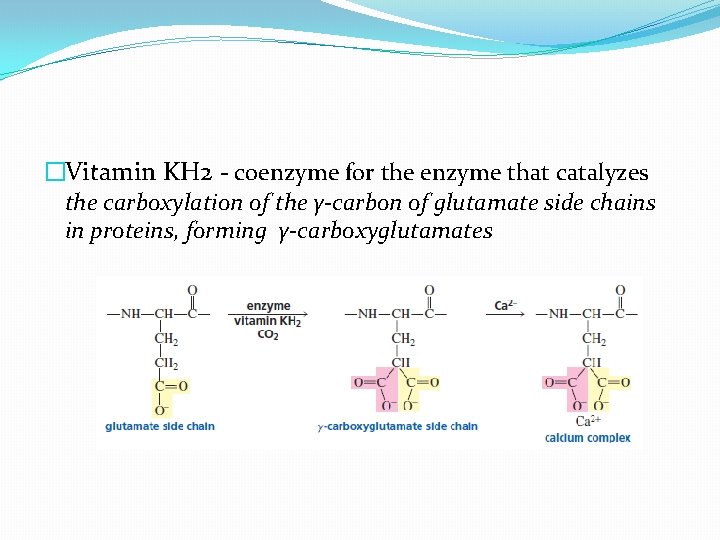
�Vitamin KH 2 - coenzyme for the enzyme that catalyzes the carboxylation of the γ-carbon of glutamate side chains in proteins, forming γ-carboxyglutamates

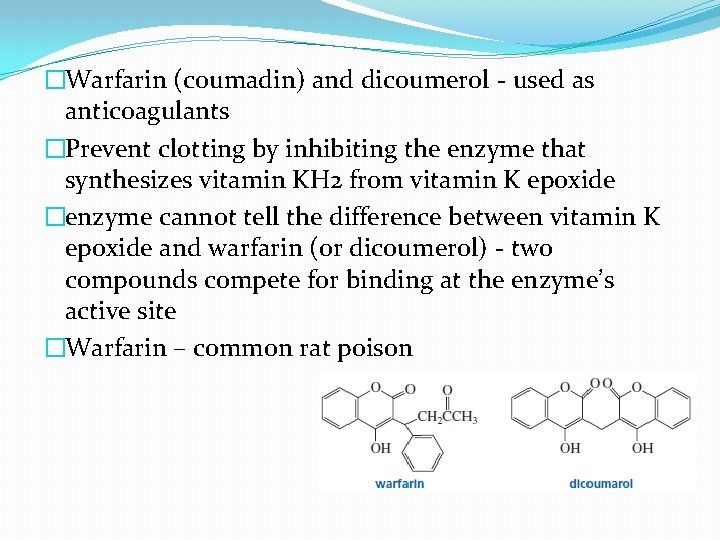
�Warfarin (coumadin) and dicoumerol - used as anticoagulants �Prevent clotting by inhibiting the enzyme that synthesizes vitamin KH 2 from vitamin K epoxide �enzyme cannot tell the difference between vitamin K epoxide and warfarin (or dicoumerol) - two compounds compete for binding at the enzyme’s active site �Warfarin – common rat poison

Problems to solve �Page 524

Summary �Enzymes �Substrate �Active site �Coenzyme �Vitamins �NAD+ and NADH �FAD and FADH 2 �TPP �Biotin �PLP �Coenzyme B 12 �THF �Vitamin KH 2