How does the collision theory help to explain











- Slides: 41

How does the collision theory help to explain why reactions occur?

Kinetics: The study of how fast a reaction happens Describes the rate of change in the concentrations of the reactants and products in a chemical reaction.

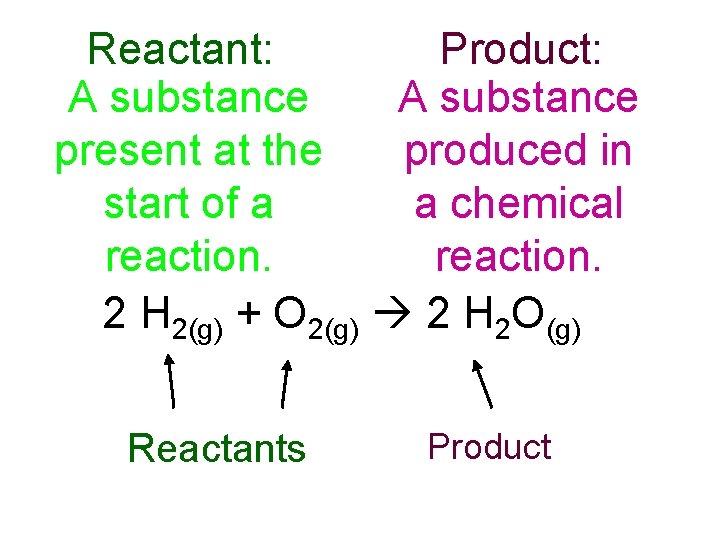
Reactant: Product: A substance present at the produced in start of a a chemical reaction. 2 H 2(g) + O 2(g) 2 H 2 O(g) Reactants Product

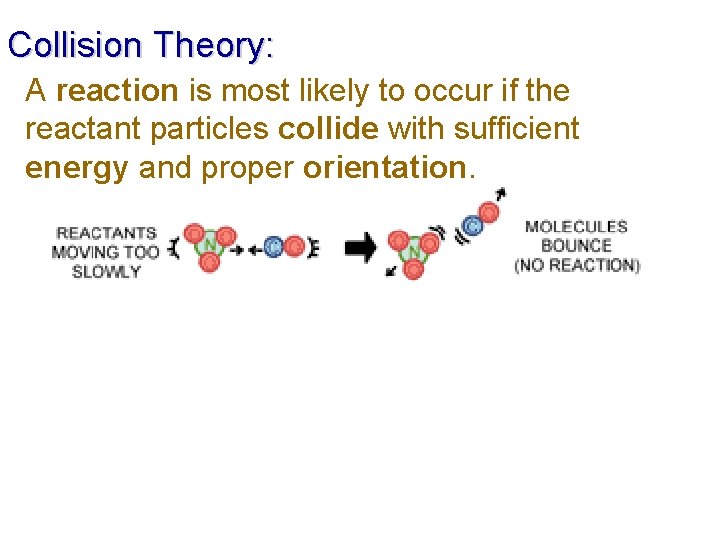
Collision Theory: A reaction is most likely to occur if the reactant particles collide with sufficient energy and proper orientation.

Factors that Affect the Rate of a Reaction:

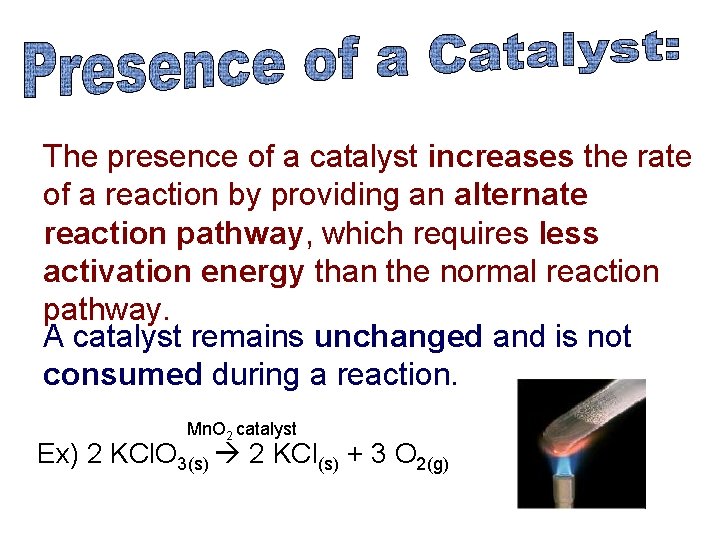
The presence of a catalyst increases the rate of a reaction by providing an alternate reaction pathway, which requires less activation energy than the normal reaction pathway. A catalyst remains unchanged and is not consumed during a reaction. Mn. O 2 catalyst Ex) 2 KCl. O 3(s) 2 KCl(s) + 3 O 2(g)

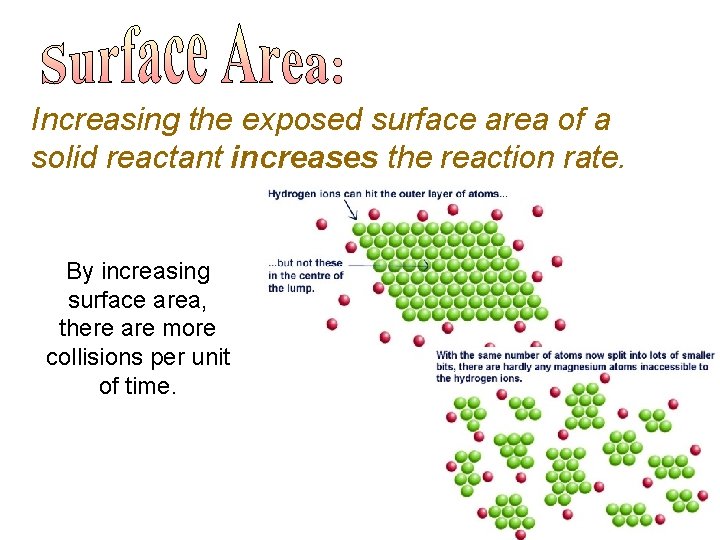
Increasing the exposed surface area of a solid reactant increases the reaction rate. By increasing surface area, there are more collisions per unit of time.

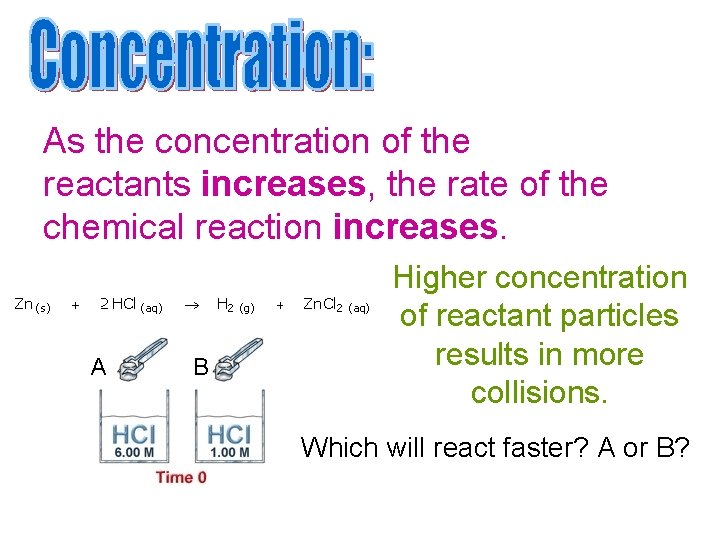
As the concentration of the reactants increases, the rate of the chemical reaction increases. A B Higher concentration of reactant particles results in more collisions. Which will react faster? A or B?

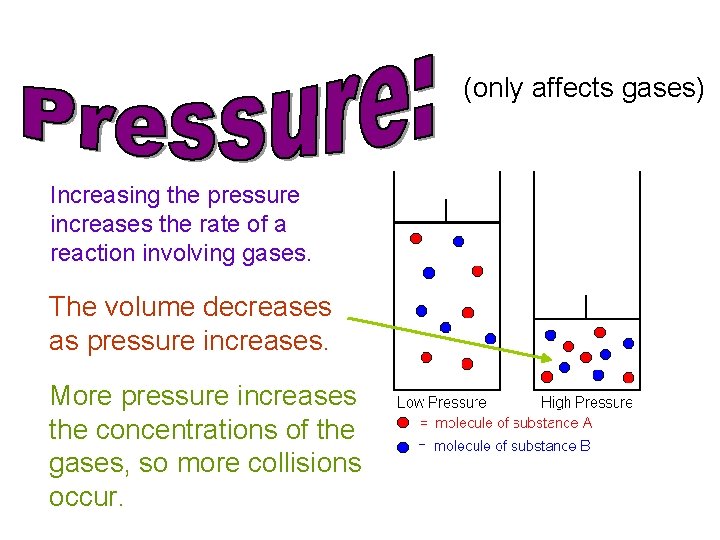
(only affects gases) Increasing the pressure increases the rate of a reaction involving gases. The volume decreases as pressure increases. More pressure increases the concentrations of the gases, so more collisions occur.

As temperature increases, the rate of a chemical reaction increases. At higher temperatures, particles collide more frequently and with more energy.

• Ions dissolved in water react the fastest: *look for (aq) • Gases tend to react faster than solids or liquids • Ionic compounds tend to react faster than covalent compounds

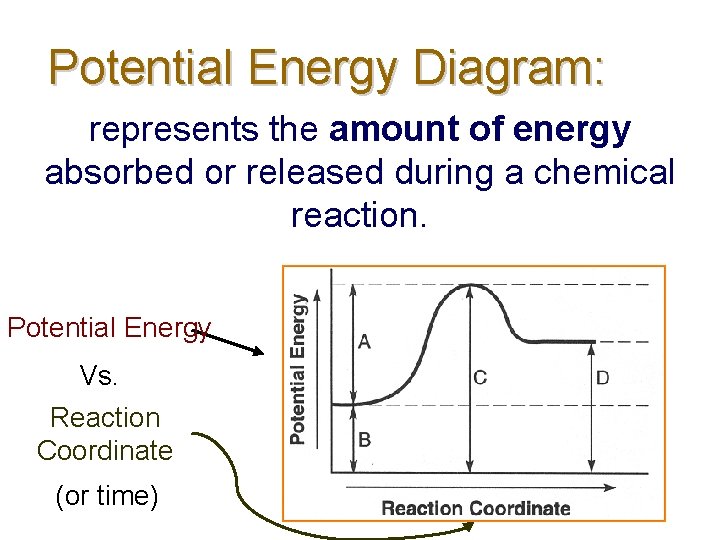
Potential Energy Diagram: represents the amount of energy absorbed or released during a chemical reaction. Potential Energy Vs. Reaction Coordinate (or time)

Potential Energy Diagram 90. PE (k. J/mol) 50. 30. Time (min)

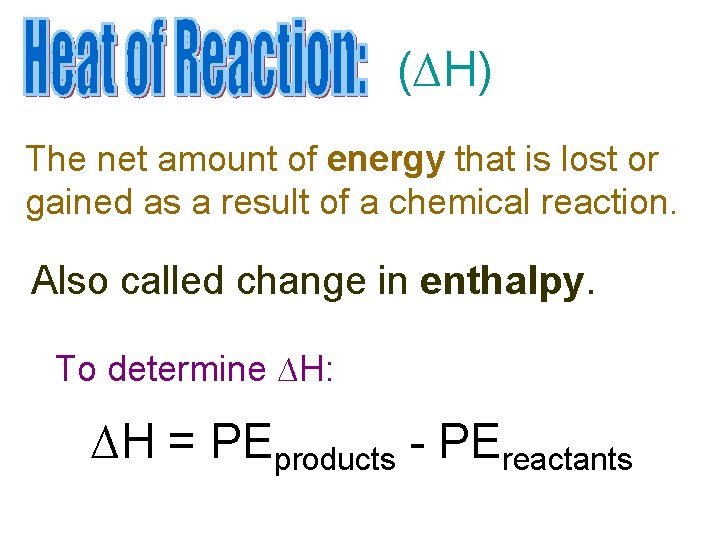
(∆H) The net amount of energy that is lost or gained as a result of a chemical reaction. Also called change in enthalpy. To determine ∆H: ∆H = PEproducts - PEreactants

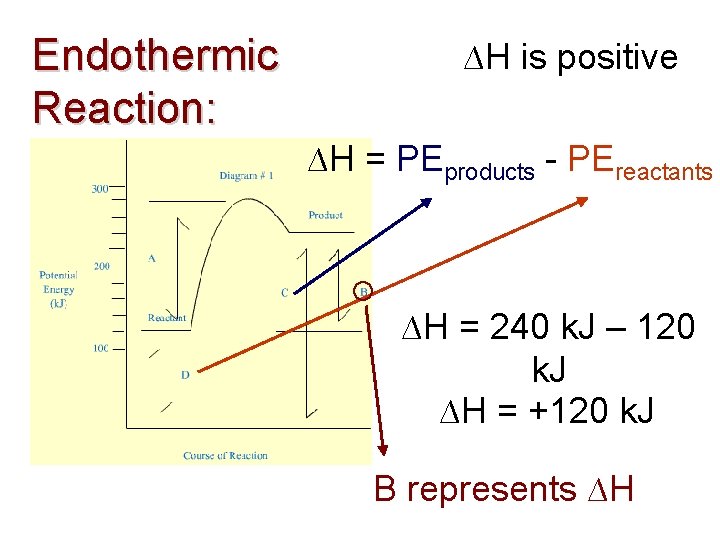
Endothermic Reaction: ∆H is positive ∆H = PEproducts - PEreactants ∆H = 240 k. J – 120 k. J ∆H = +120 k. J B represents ∆H

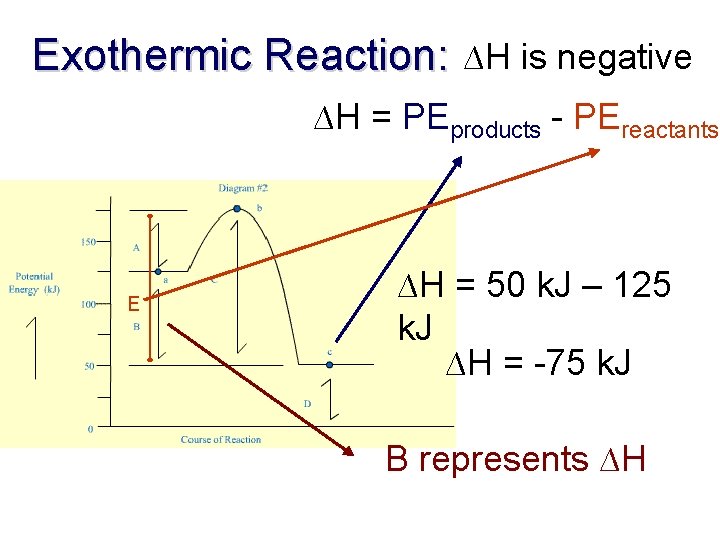
Exothermic Reaction: ∆H is negative ∆H = PEproducts - PEreactants E ∆H = 50 k. J – 125 k. J ∆H = -75 k. J B represents ∆H

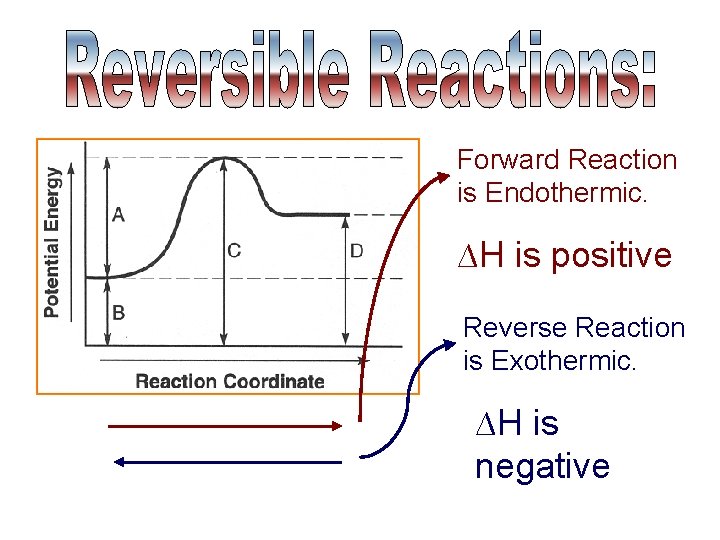
Forward Reaction is Endothermic. ∆H is positive Reverse Reaction is Exothermic. ∆H is negative

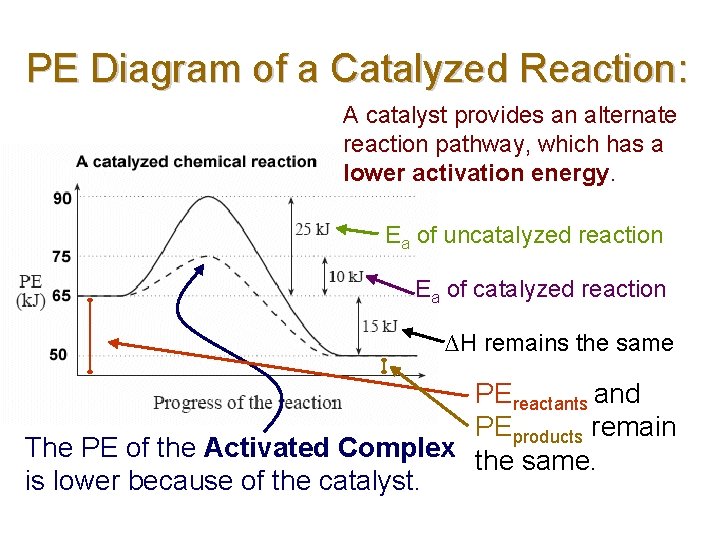
PE Diagram of a Catalyzed Reaction: A catalyst provides an alternate reaction pathway, which has a lower activation energy. Ea of uncatalyzed reaction Ea of catalyzed reaction ∆H remains the same PEreactants and PEproducts remain The PE of the Activated Complex the same. is lower because of the catalyst.

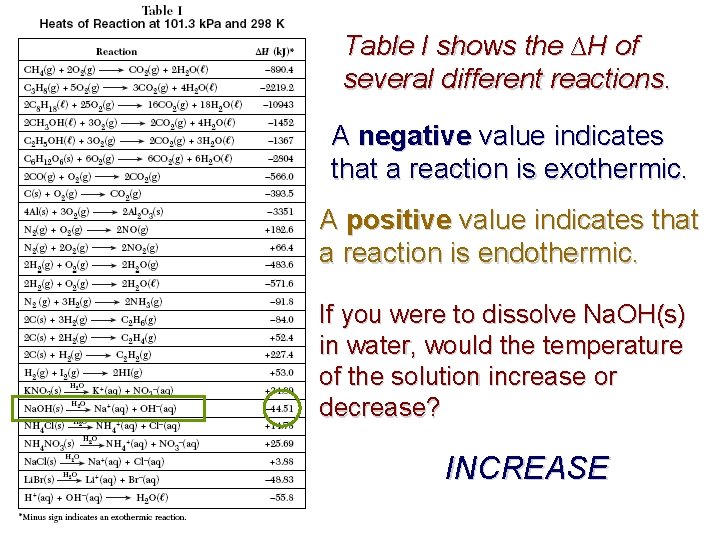
Table I shows the ∆H of several different reactions. A negative value indicates that a reaction is exothermic. A positive value indicates that a reaction is endothermic. If you were to dissolve Na. OH(s) in water, would the temperature of the solution increase or decrease? INCREASE

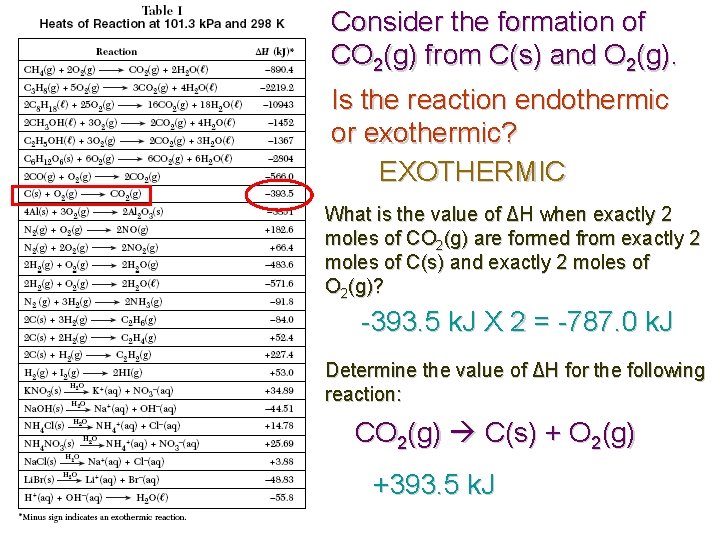
Consider the formation of CO 2(g) from C(s) and O 2(g). Is the reaction endothermic or exothermic? EXOTHERMIC What is the value of ΔH when exactly 2 moles of CO 2(g) are formed from exactly 2 moles of C(s) and exactly 2 moles of O 2(g)? -393. 5 k. J X 2 = -787. 0 k. J Determine the value of ΔH for the following reaction: CO 2(g) C(s) + O 2(g) +393. 5 k. J

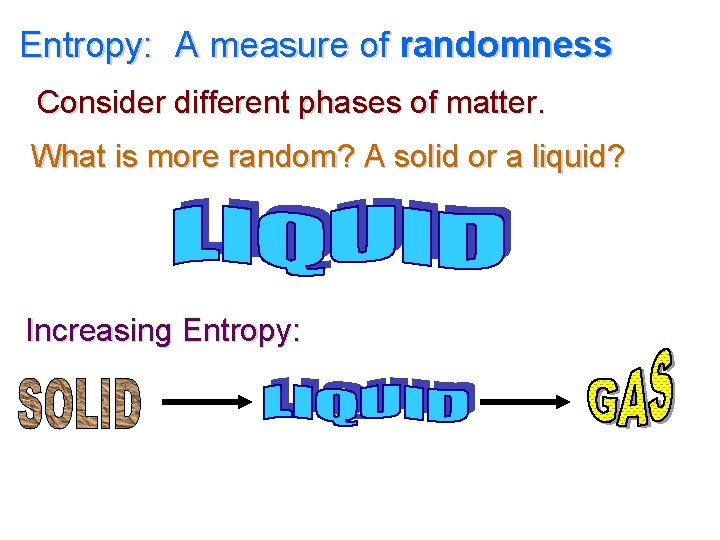
Entropy: A measure of randomness Consider different phases of matter. What is more random? A solid or a liquid? Increasing Entropy:

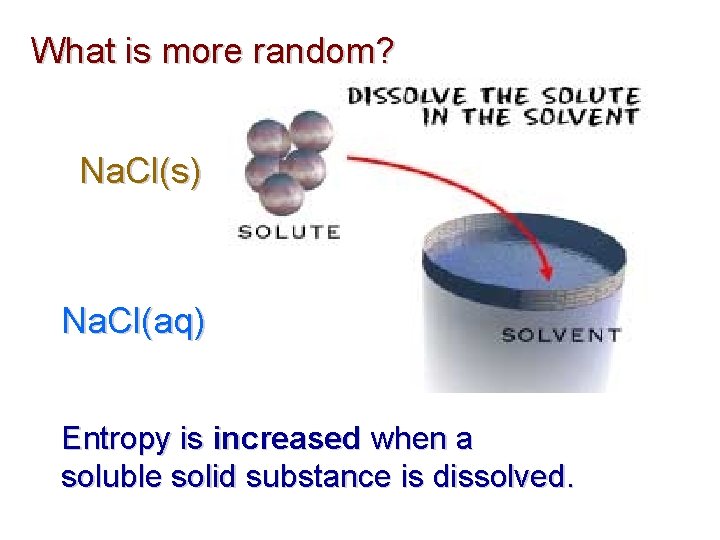
What is more random? Na. Cl(s) Na. Cl(aq) Entropy is increased when a soluble solid substance is dissolved.

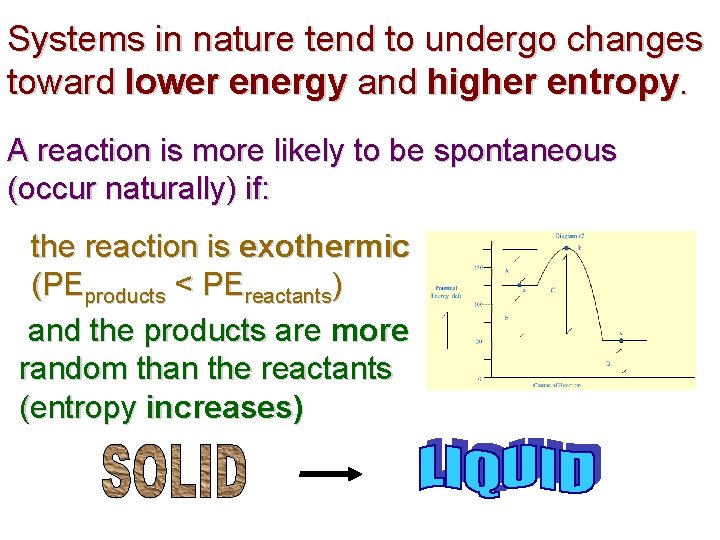
Systems in nature tend to undergo changes toward lower energy and higher entropy. A reaction is more likely to be spontaneous (occur naturally) if: the reaction is exothermic (PEproducts < PEreactants) and the products are more random than the reactants (entropy increases)

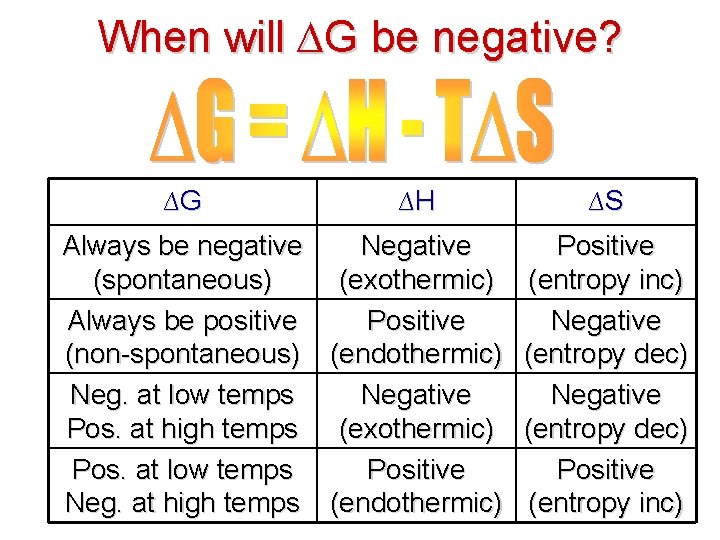
Gibbs Free Energy Equation: Mathematically explains when reactions will occur spontaneously A reaction is spontaneous if the value of ∆G is NEGATIVE!

When will ∆G be negative? ∆G ∆H ∆S Always be negative Negative Positive (spontaneous) (exothermic) (entropy inc) Always be positive Positive Negative (non-spontaneous) (endothermic) (entropy dec) Neg. at low temps Negative Pos. at high temps (exothermic) (entropy dec) Pos. at low temps Positive Neg. at high temps (endothermic) (entropy inc)

EQUAL RATES The rate of the forward reaction equals the rate of the reverse reaction. The concentration of the reactants remain constant. The concentration of the products remain constant. (NOT NECESSARILY EQUAL TO EACH OTHER!!)

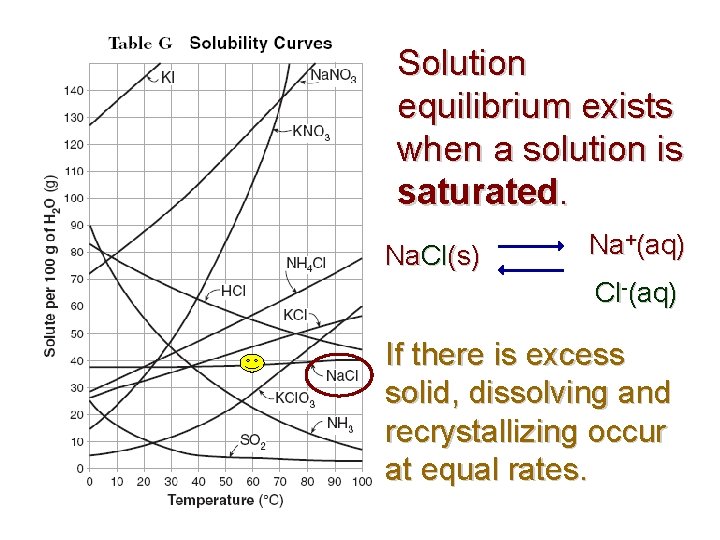
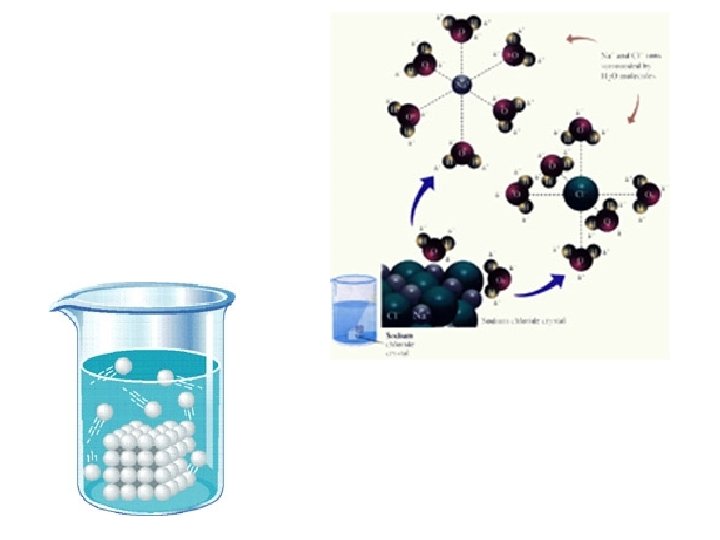
Solution equilibrium exists when a solution is saturated. Na. Cl(s) Na+(aq) Cl-(aq) If there is excess solid, dissolving and recrystallizing occur at equal rates.


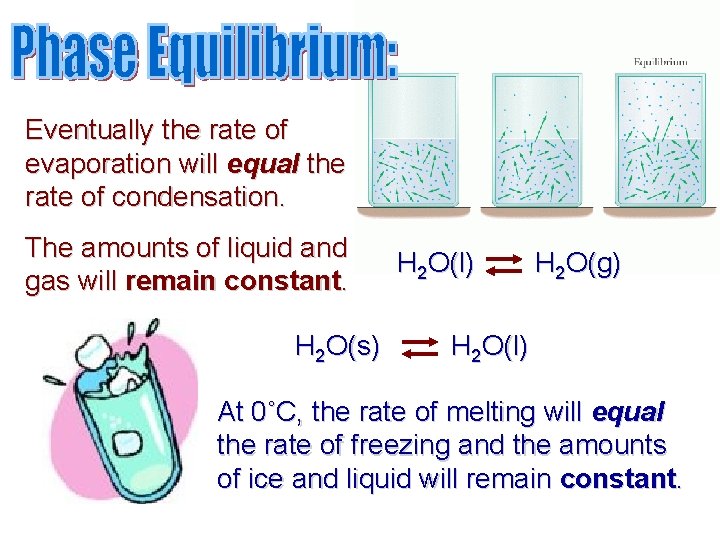
Eventually the rate of evaporation will equal the rate of condensation. The amounts of liquid and gas will remain constant. H 2 O(s) H 2 O(l) H 2 O(g) H 2 O(l) At 0˚C, the rate of melting will equal the rate of freezing and the amounts of ice and liquid will remain constant.

Le. Chatelier’s Principle: If a stress is applied to a system at equilibrium, the equilibrium will shift to reduce or alleviate the stress. N 2(g) +3 H 2(g) 2 NH 3(g) + 91. 8 k. J

Stresses that will affect a system at equilibrium: • Concentration • Pressure and Volume (gases) • Temperature

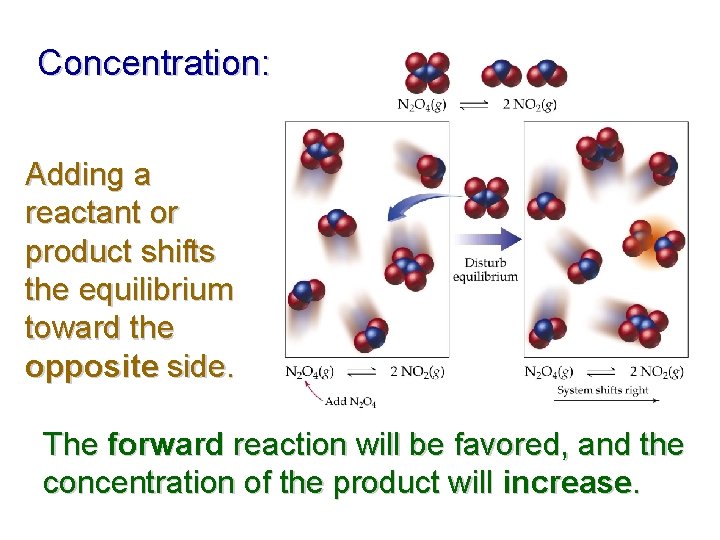
Concentration: Adding a reactant or product shifts the equilibrium toward the opposite side. The forward reaction will be favored, and the concentration of the product will increase.

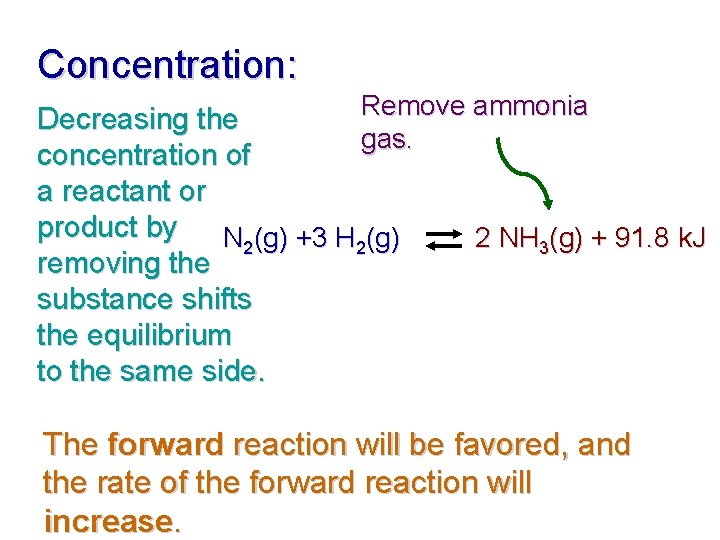
Concentration: Remove ammonia gas. Decreasing the concentration of a reactant or product by N 2(g) +3 H 2(g) removing the substance shifts the equilibrium to the same side. 2 NH 3(g) + 91. 8 k. J The forward reaction will be favored, and the rate of the forward reaction will increase.

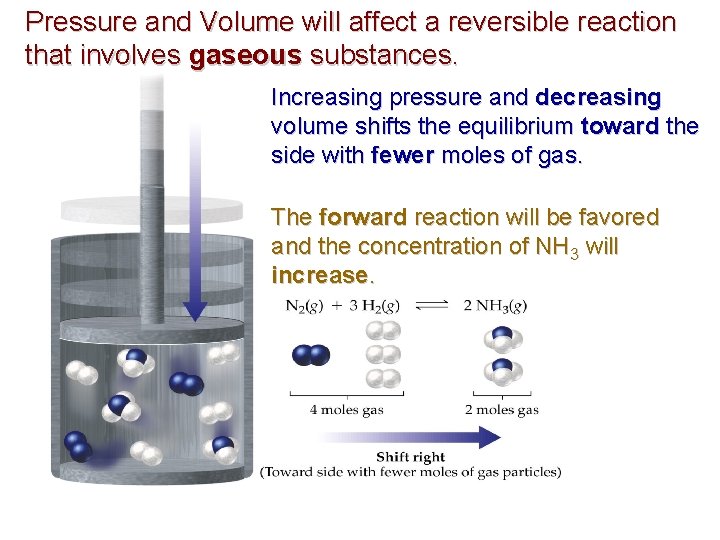
Pressure and Volume will affect a reversible reaction that involves gaseous substances. Increasing pressure and decreasing volume shifts the equilibrium toward the side with fewer moles of gas. The forward reaction will be favored and the concentration of NH 3 will increase.

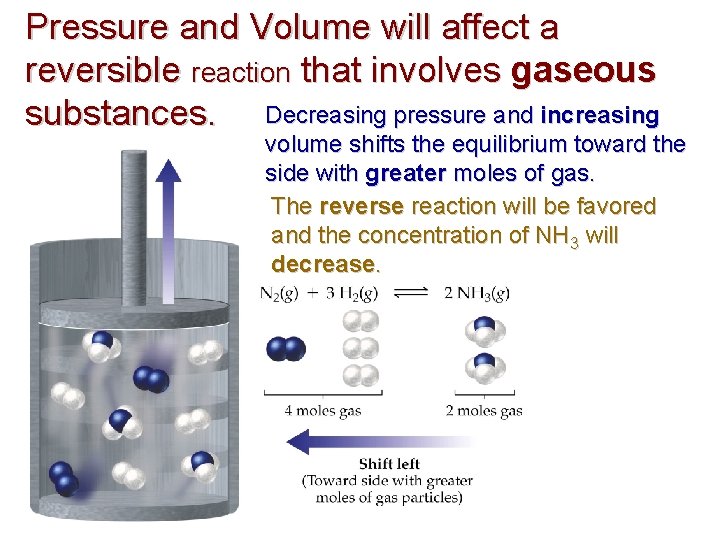
Pressure and Volume will affect a reversible reaction that involves gaseous substances. Decreasing pressure and increasing volume shifts the equilibrium toward the side with greater moles of gas. The reverse reaction will be favored and the concentration of NH 3 will decrease.

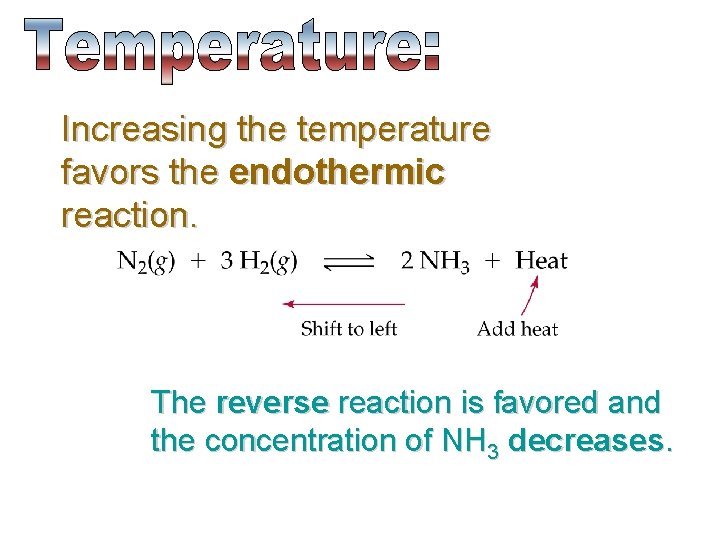
Increasing the temperature favors the endothermic reaction. The reverse reaction is favored and the concentration of NH 3 decreases.

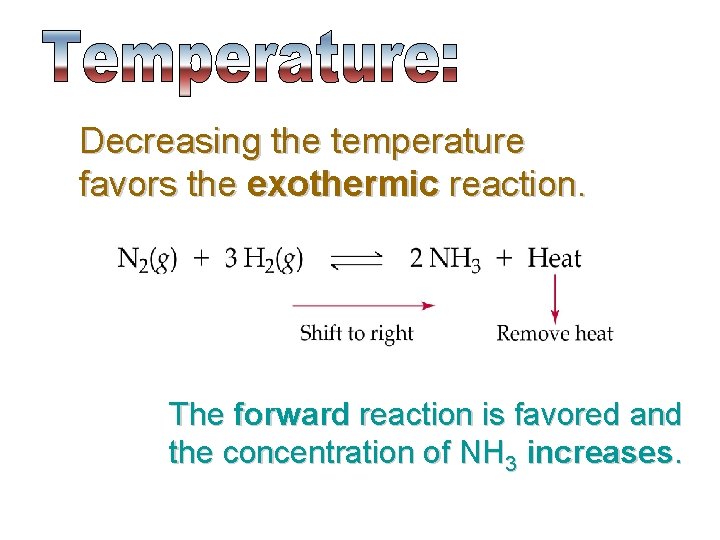
Decreasing the temperature favors the exothermic reaction. The forward reaction is favored and the concentration of NH 3 increases.

Adding a Catalyst • Does not cause the equilibrium to shift • The catalyst increases the rates of both forward and reverse reactions equally • The rates after the increase are still equal to each other

(Aqueous solutions) The solubility of an ionic substance decreases when a common is added to a saturated solution. Consider the following saturated solution: Ag. Cl(s) Ag+(aq) + Cl-(aq) What happens to the equilibrium when Na. Cl(s) is added? The concentration of Cl-(aq)increased because Na. Cl (s) dissociated into Na+(aq) and Cl-(aq).

Ag. Cl(s) Ag+(aq) + Cl-(aq) Na. Cl(s) is added The concentration of Cl-(aq) is increased Think of La. Chatelier’s Principle: In which direction will the equilibrium shift? What will happen to the concentration of Ag+(aq)? What will happen to the amount of Ag. Cl(s)?

The following equilibrium has been established: KNO 3(s) K+(aq) + NO 3 -(aq) In which direction will the equilibrium shift when KI(s) is added? What will be the effect on the amounts of NO 3 -(aq) and KNO 3(s)?