How Does Salinity affect Density in the Ocean
























- Slides: 27

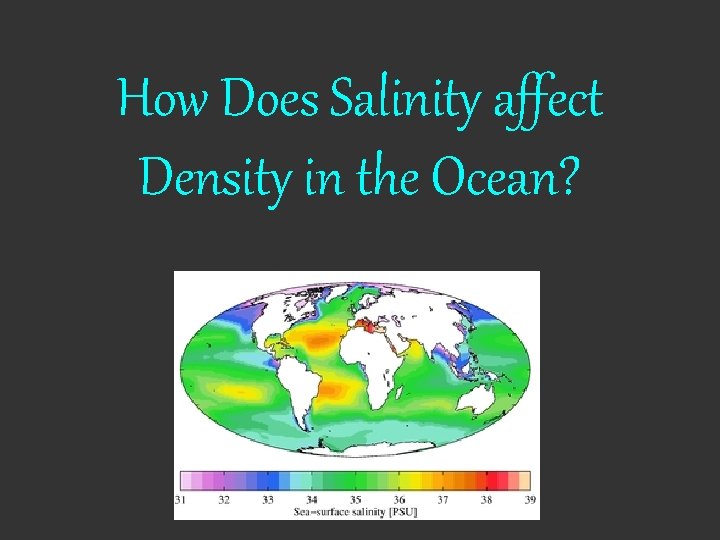
How Does Salinity affect Density in the Ocean?

What is Salinity? • The total concentration of all dissolved inorganic solids • Many salts dissolved in seawater – Na. Cl, KCl

Why is the Ocean Salty? – Weathering of rocks on land (carried by rivers into the ocean) – Waves and surf erode coastal rocks and ocean bottoms – Salt domes

How do you Measure Salinity? • Measured in parts per thousand (ppt) – Salinity is expressed by the amount of salt found in 1, 000 grams of water. • If the salinity of the ocean is 32 ppt, it means there are 32 grams of salt in 1, 000 grams of water *Hydrometer, refractometer

Ocean Salinity • Varies very little overall, but there is a great deal of variation due to location – Approx. 32 ppt – Lower salinity • areas near rivers, areas with high amounts of rainfall – High salinity • areas with a lot of evaporation/lots of sun

How does the addition of salt affect water? • Raises the boiling point • Decreases freezing temperature • Decreases heat capacity • Slows evaporation • Changes the density


Experiment • How does salinity affect density in the ocean? – Draw your observations – Describe in words what you observe – Answer the above question

How do fish adapt to changes in salinity?

Diffusion & Osmosis • Diffusion – the tendency for a liquid, gas, or solute to flow from an area of high concentration to an area of low concentration • Osmosis – diffusion of water through a semipermeable membrane

Osmoregulators • Organisms that have a regulation process that allows them to adjust the water concentration within their cells

Osmoconformers • Organisms that cannot control their internal water concentration – Their internal salinity rises and falls with the surrounding environment • Example: marine invertebrates

Descriptors • Euryhaline (“Eury” – wide, “haline” – salt) – Can tolerate significant fluctuations • Example: salmon & manatees • Stenohaline (“Steno” – narrow) – Cannot tolerate significant fluctuations • Example: goldfish & grouper

Video Clips • Dead Sea Dying • Bowling Ball


How does Temperature Affect Density in the ocean?

Experiment • What is the effect of temperature on density? • How does this relate to the ocean? – Draw your observations – Describe in words what you observe – Answer the above questions

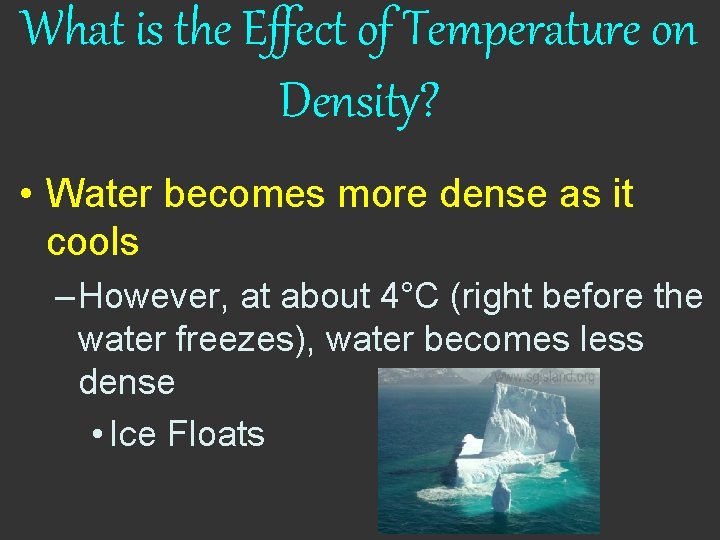
What is the Effect of Temperature on Density? • Water becomes more dense as it cools – However, at about 4°C (right before the water freezes), water becomes less dense • Ice Floats

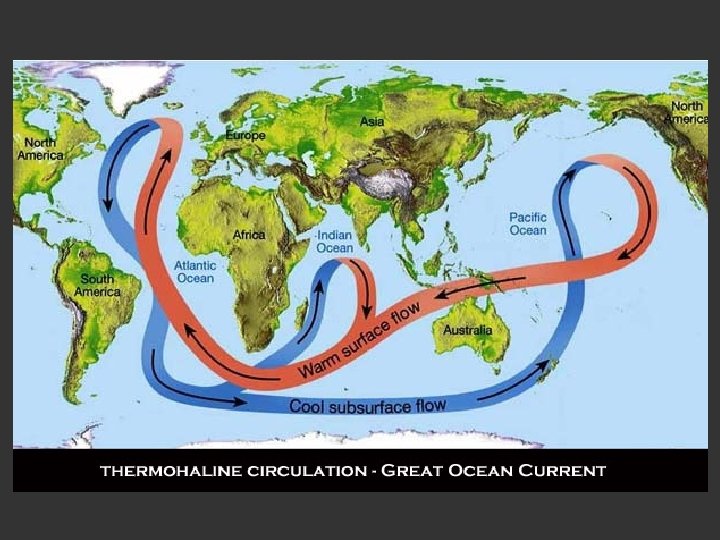
Ocean Water Density • Because temp and salinity affect water density, seawater stratifies (forms layers) – Thermocline: an abrupt change in temp leading to 2 distinct layers – Halocline: an abrupt change in salinity leading to 2 distinct layers – Pycnocline: Thermocline and halocline together creating a boundary between layers of differing water density


Experiment: Layering Liquids Using what you have learned this week, can you use the 4 liquids to show stratified layers without mixing?

Key • Red: Hot and Salty • Blue: Cold and Salty • Yellow: Hot and Fresh • Green: Cold and Fresh

Conclusions: • Answer the questions given to you on a separate piece of paper in complete sentences!


National Geographic Article • Read the article individually • Answer the questions as a group • Nutrients in the Ocean: – Phosphate, Nitrate, Silica, Iron…things typically dissolved in the ocean that plants and animals need to survive

• Describe the importance of the ocean circulation pattern in the Southern Hemisphere. • How do differences in ocean water density make the circulation pattern possible? • According to the article, which has a greater impact on density in the ocean, salinity or temperature? Suggest a possible reason for this. • Define Biological Productivity • How might global warming affect the conveyor belt?
