How do you know if a chemical reaction





![Table #1: Round #1 • Reactant Person: Rate = ½[40] = 20 • Product Table #1: Round #1 • Reactant Person: Rate = ½[40] = 20 • Product](https://slidetodoc.com/presentation_image_h2/696a8b6a2f9c9be1f8d2399eafb39cd7/image-9.jpg)
![Table #1: Round #2 • Reactant Person: Rate = ½[20] = 10 • Product Table #1: Round #2 • Reactant Person: Rate = ½[20] = 10 • Product](https://slidetodoc.com/presentation_image_h2/696a8b6a2f9c9be1f8d2399eafb39cd7/image-11.jpg)



- Slides: 31

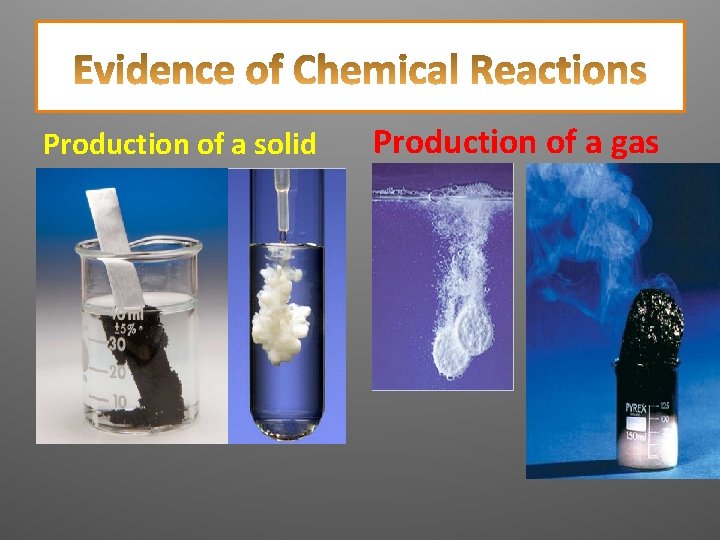
How do you know if a chemical reaction is happening? List at least four observations that would indicate a chemical reaction is taking place.

Color Change Energy change (heat/light) )

Production of a solid Production of a gas

Do reactions only procede forward (R → P) or can they go also go in reverse (P → R)? Link to Chemical Oscillator • • Reversible reaction • Link to phet reversible • REACTIONS CAN GO IN BOTH THE FORWARD AND REVERSE DIRECTIONS!

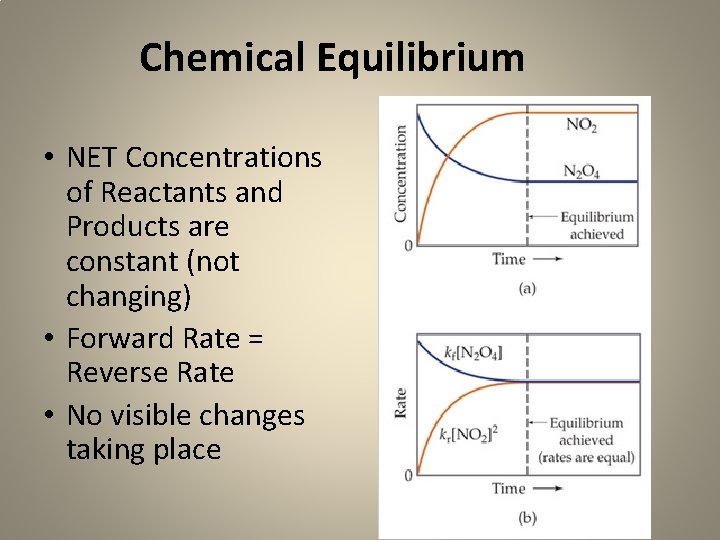
Will these chemical reactions run forever? Link to Rings of Color Link to Briggs-Rausher NO! ALL CHEMICAL REACTIONS IN A CLOSED SYSTEM WILL EVENTUALLY REACH CHEMICAL EQUILIBRIUM

• NO Color CHANGES • NO Temperature CHANGE • NO solid or gases products appearing

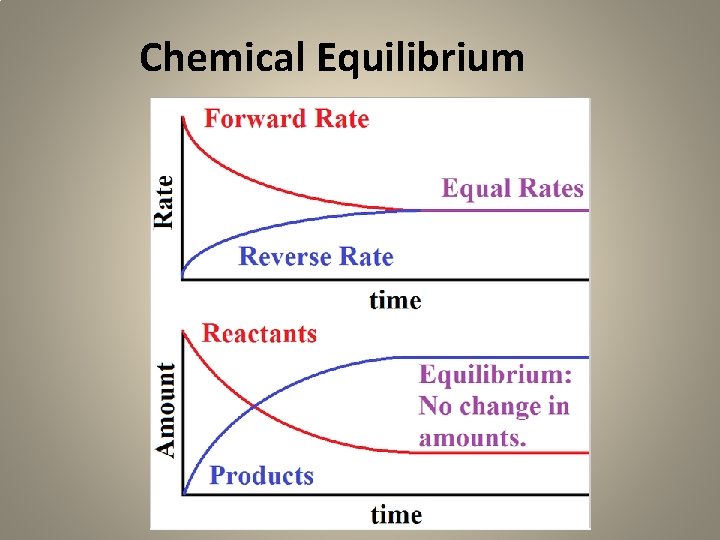
Chemical Equilibrium • NET Concentrations of Reactants and Products are constant (not changing) • Forward Rate = Reverse Rate • No visible changes taking place

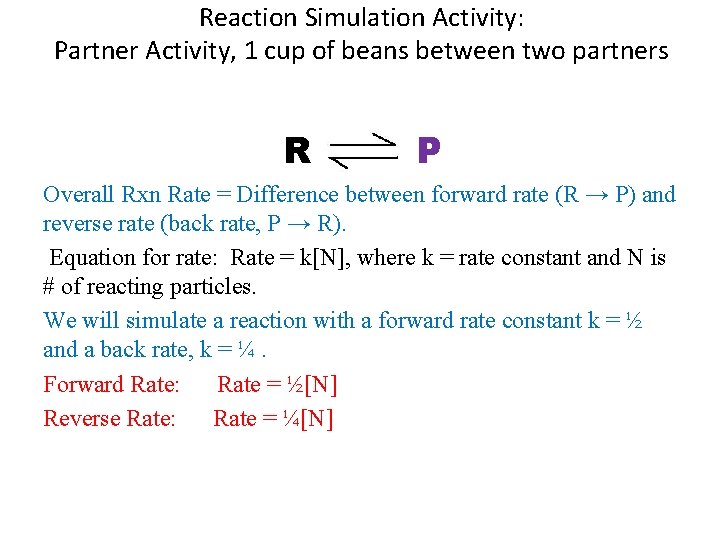
Reaction Simulation Activity: Partner Activity, 1 cup of beans between two partners R P Overall Rxn Rate = Difference between forward rate (R → P) and reverse rate (back rate, P → R). Equation for rate: Rate = k[N], where k = rate constant and N is # of reacting particles. We will simulate a reaction with a forward rate constant k = ½ and a back rate, k = ¼. Forward Rate: Rate = ½[N] Reverse Rate: Rate = ¼[N]
![Table 1 Round 1 Reactant Person Rate ½40 20 Product Table #1: Round #1 • Reactant Person: Rate = ½[40] = 20 • Product](https://slidetodoc.com/presentation_image_h2/696a8b6a2f9c9be1f8d2399eafb39cd7/image-9.jpg)
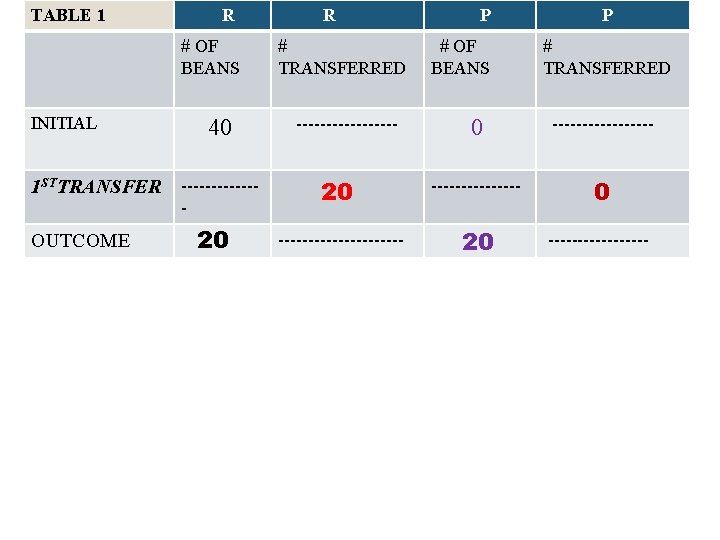
Table #1: Round #1 • Reactant Person: Rate = ½[40] = 20 • Product Person: Rate = ¼[0] = 0 • Reactant person transfer 20 to product person; product person transfer zero back; Each partner count total and record results in outcome.

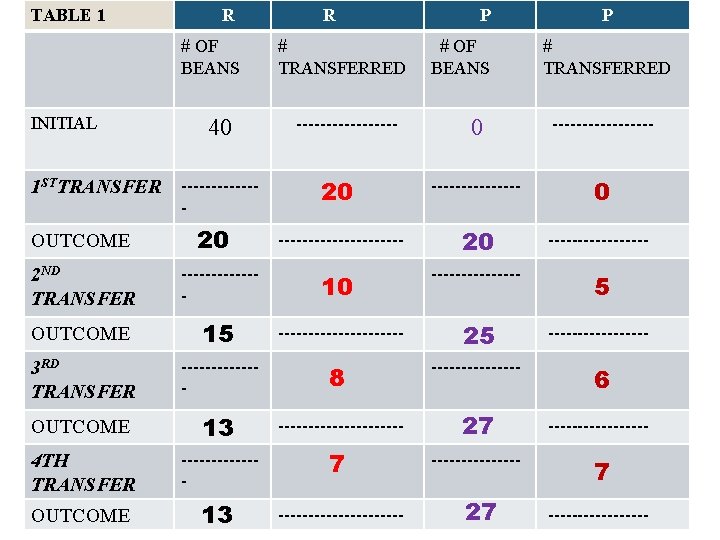
TABLE 1 R # OF BEANS INITIAL 1 STTRANSFER OUTCOME 40 ------- 20 R # TRANSFERRED --------- P # OF BEANS P # TRANSFERRED 0 --------- 20 ----------- 20 ---------
![Table 1 Round 2 Reactant Person Rate ½20 10 Product Table #1: Round #2 • Reactant Person: Rate = ½[20] = 10 • Product](https://slidetodoc.com/presentation_image_h2/696a8b6a2f9c9be1f8d2399eafb39cd7/image-11.jpg)
Table #1: Round #2 • Reactant Person: Rate = ½[20] = 10 • Product Person: Rate = ¼[20] = 5 • Reactant person transfer 10 to product person; product person transfer 5 back; Each partner count total and record results in outcome. • Work through rounds 3 & 4 on your own. Round fractions. • Example #1: 15 ÷ 2 = 7. 5 → 8 • Example #2: 25 ÷ 4 = 6. 25 → 6

TABLE 1 R # OF BEANS INITIAL 1 STTRANSFER OUTCOME 40 ------- 20 R # TRANSFERRED --------- P # OF BEANS P # TRANSFERRED 0 --------- 20 ----------- 20 --------- 5 2 ND TRANSFER ------- 10 -------- OUTCOME 15 ----------- 25 --------- 3 RD TRANSFER ------- 8 -------- 6 OUTCOME 13 ----------- 27 --------- 4 TH TRANSFER ------- 7 -------- 7 OUTCOME 13 ----------- 27 ---------

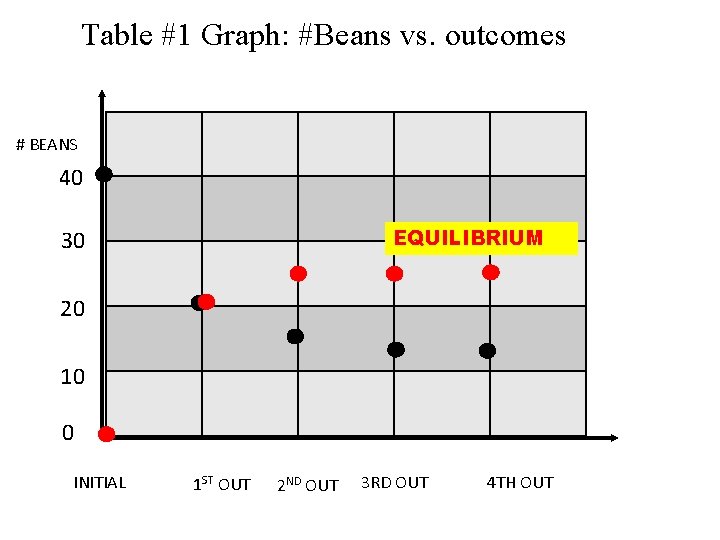
Table #1 Graph: #Beans vs. outcomes # BEANS 40 EQUILIBRIUM 30 20 10 0 INITIAL 1 ST OUT 2 ND OUT 3 RD OUT 4 TH OUT

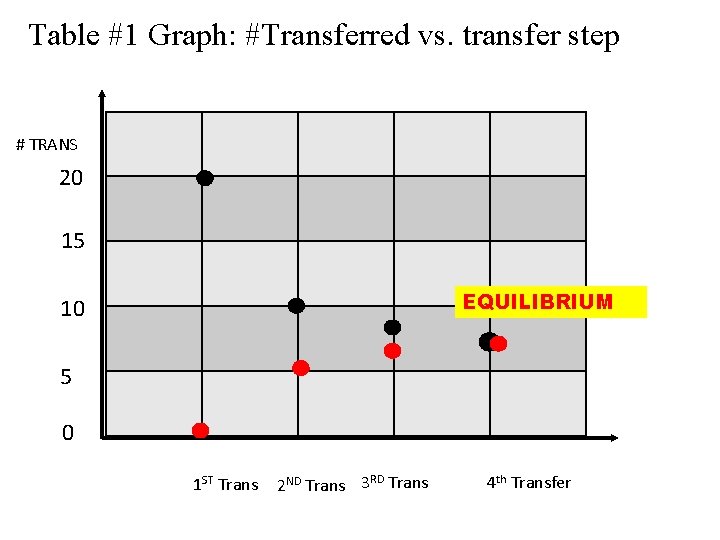
Table #1 Graph: #Transferred vs. transfer step # TRANS 20 15 EQUILIBRIUM 10 5 0 1 ST Trans 2 ND Trans 3 RD Trans 4 th Transfer

Chemical Equilibrium

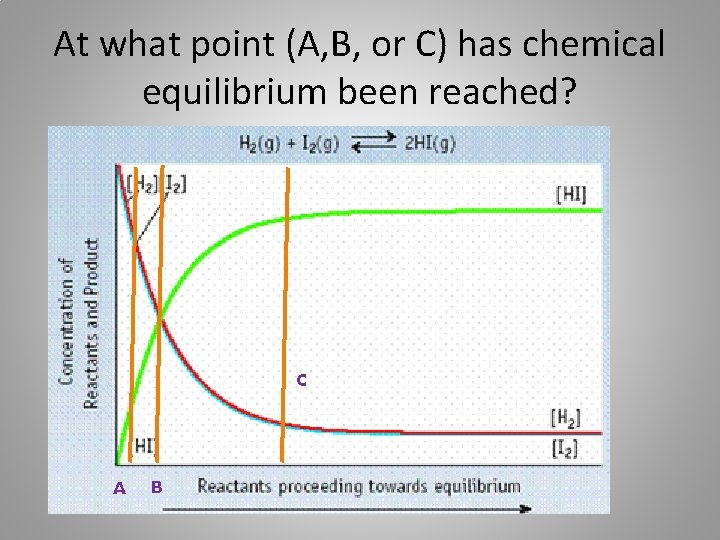
At what point (A, B, or C) has chemical equilibrium been reached? C A B

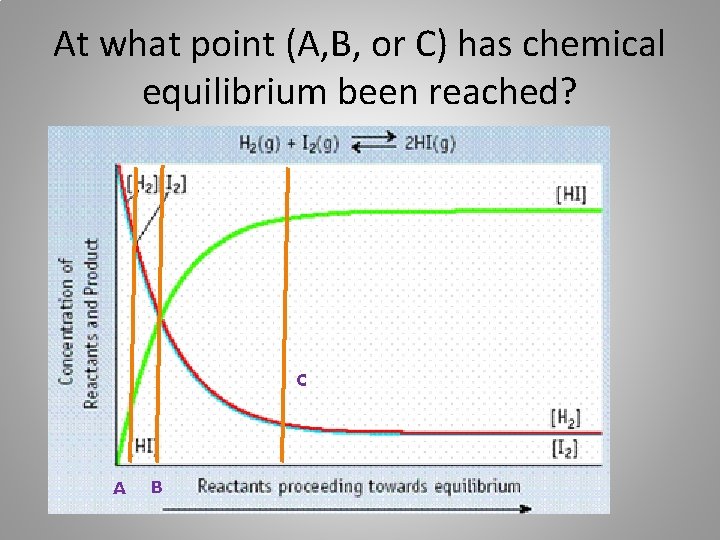
At what point (A, B, or C) has chemical equilibrium been reached? C A B

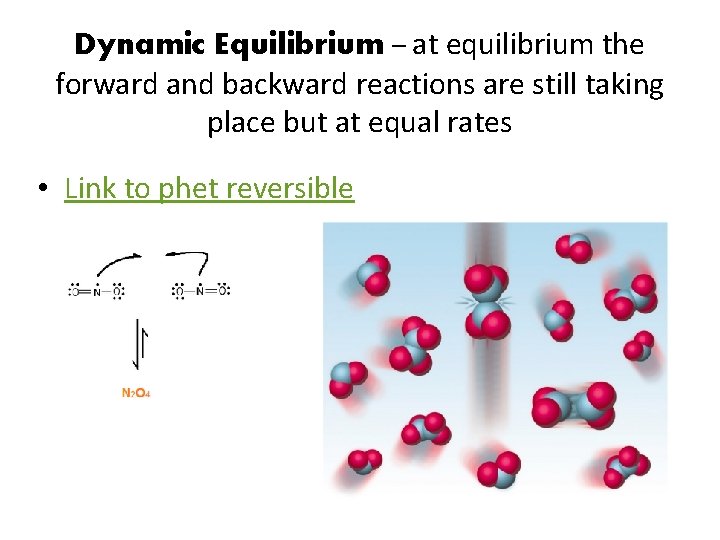
Dynamic Equilibrium – at equilibrium the forward and backward reactions are still taking place but at equal rates • Link to phet reversible

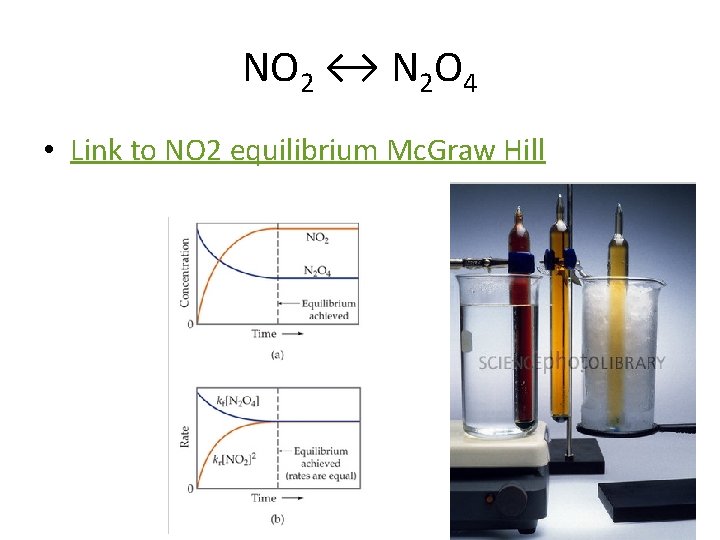
NO 2 ↔ N 2 O 4 • Link to NO 2 equilibrium Mc. Graw Hill

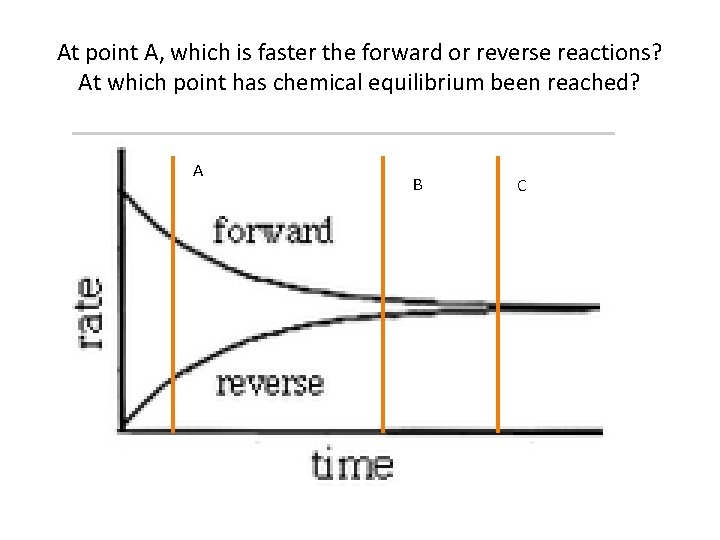
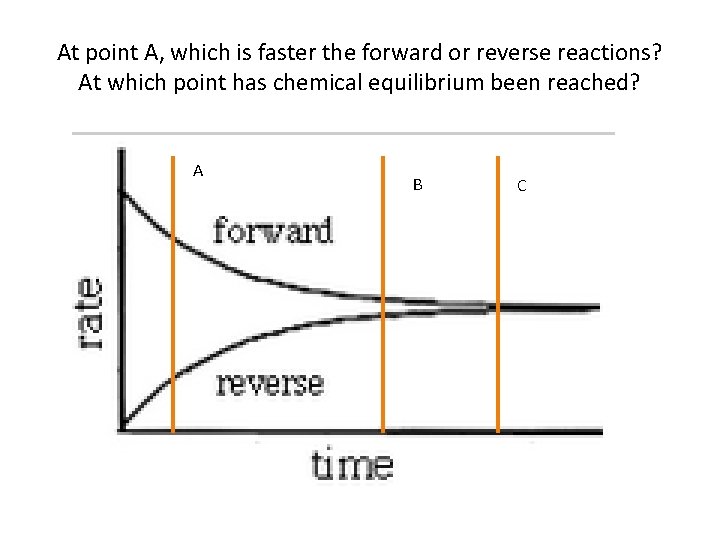
At point A, which is faster the forward or reverse reactions? At which point has chemical equilibrium been reached? A B C

At point A, which is faster the forward or reverse reactions? At which point has chemical equilibrium been reached? A B C

HW 11 -3, p. 543, #3 -12 (WB, p. 100) • 3) How do chemists envision reactions taking place in terms of a collision model For reactions? Give an example of a simple reaction and how you might envision the reaction taking place by means of collisions between molecules.

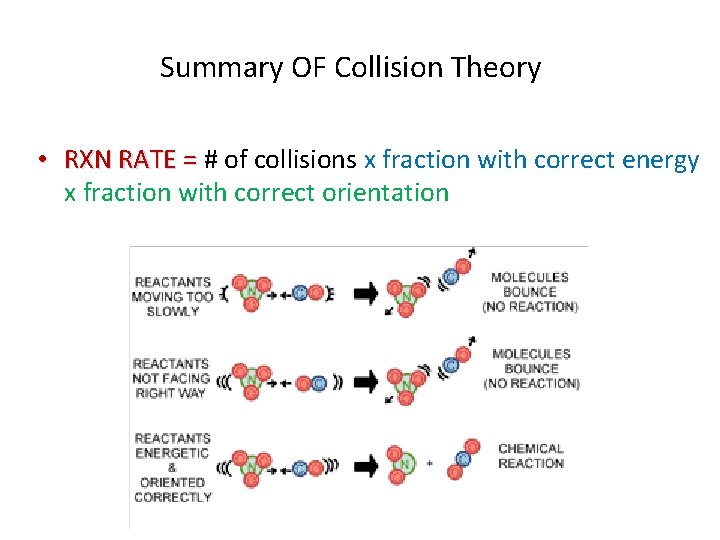
Summary OF Collision Theory • RXN RATE = # of collisions x fraction with correct energy x fraction with correct orientation

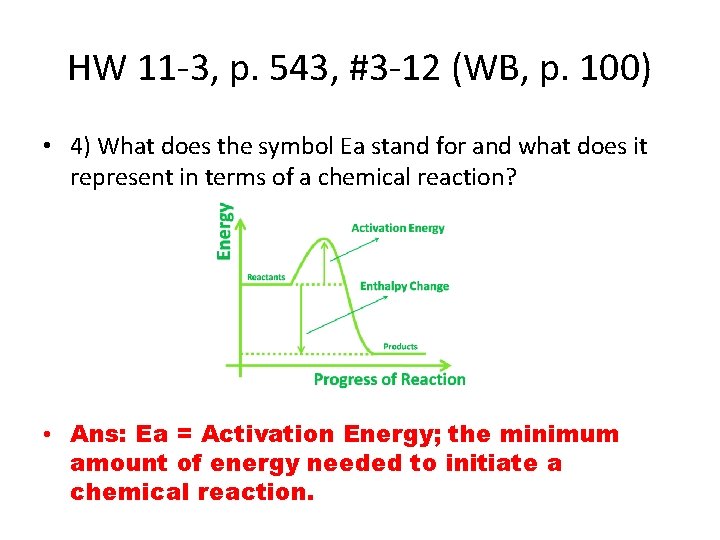
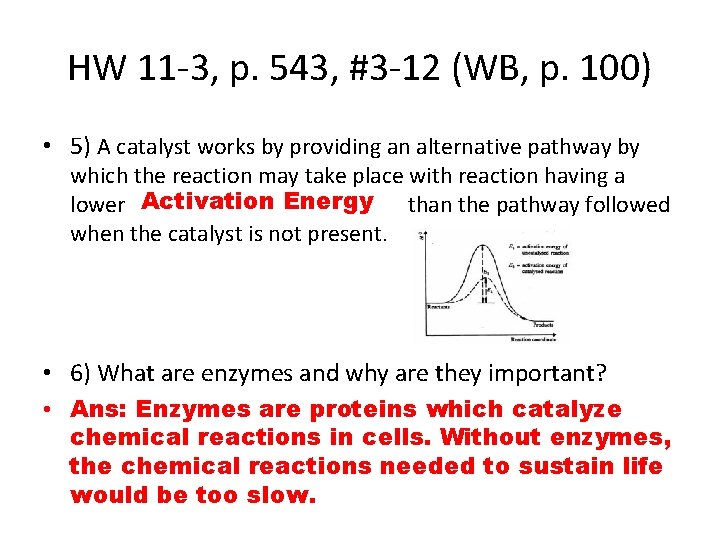
HW 11 -3, p. 543, #3 -12 (WB, p. 100) • 4) What does the symbol Ea stand for and what does it represent in terms of a chemical reaction? • Ans: Ea = Activation Energy; the minimum amount of energy needed to initiate a chemical reaction.

HW 11 -3, p. 543, #3 -12 (WB, p. 100) • 5) A catalyst works by providing an alternative pathway by which the reaction may take place with reaction having a lower Activation Energy than the pathway followed when the catalyst is not present. • 6) What are enzymes and why are they important? • Ans: Enzymes are proteins which catalyze chemical reactions in cells. Without enzymes, the chemical reactions needed to sustain life would be too slow.

HW 11 -3, p. 543, #3 -12 (WB, p. 100) • 7) How does equilibrium represent a balancing of opposing processes? Give an example of an “equilibrium” encountered in everyday life, showing how the opposing processes oppose each other. • Possible Answer: If population of a town is 100, 000 at the beginning and end of a year, best explanation is an equal # of people moved in and out of town, not that no one moved in or out during an entire year.

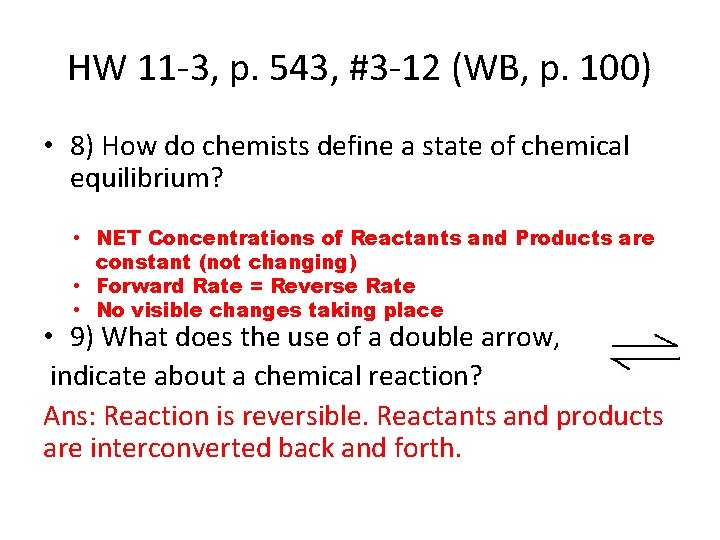
HW 11 -3, p. 543, #3 -12 (WB, p. 100) • 8) How do chemists define a state of chemical equilibrium? • NET Concentrations of Reactants and Products are constant (not changing) • Forward Rate = Reverse Rate • No visible changes taking place • 9) What does the use of a double arrow, indicate about a chemical reaction? Ans: Reaction is reversible. Reactants and products are interconverted back and forth.

HW 11 -3, p. 543, #3 -12 (WB, p. 100) • 11) What does it mean to say that a chemical or physical equilibrium is dynamic? • Answer: The forward and reverse reactions are still taking place, but at equal rates.

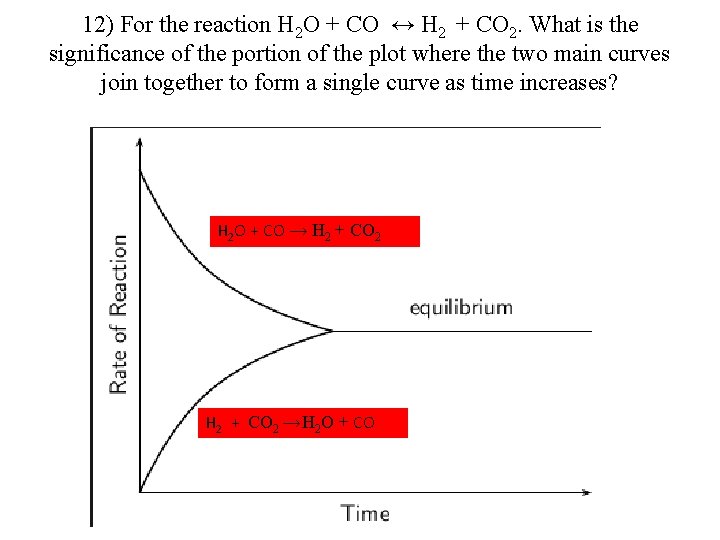
12) For the reaction H 2 O + CO ↔ H 2 + CO 2. What is the significance of the portion of the plot where the two main curves join together to form a single curve as time increases? H 2 O + CO → H 2 + CO 2 → H 2 O + CO

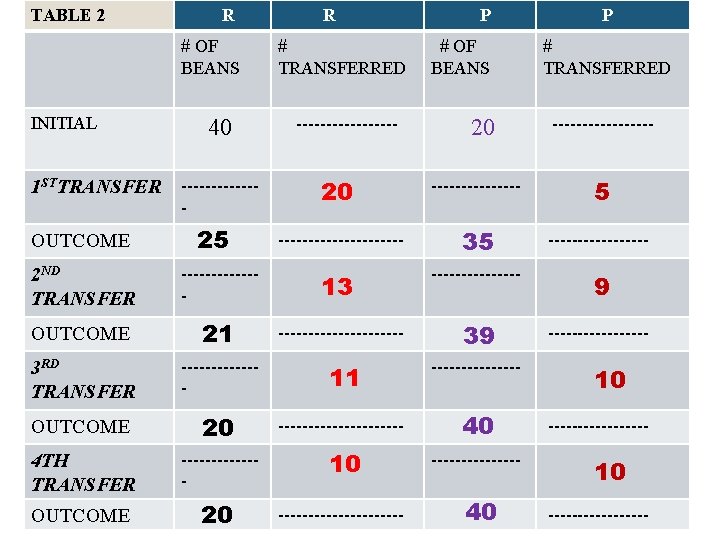
TABLE 2 R # OF BEANS INITIAL 1 STTRANSFER OUTCOME 40 ------- 25 R # TRANSFERRED --------- P # OF BEANS 20 P # TRANSFERRED --------- 20 -------- 5 ----------- 35 --------- 9 --------- 2 ND TRANSFER ------- 13 -------- OUTCOME 21 ----------- 39 3 RD TRANSFER ------- 11 -------- OUTCOME 20 ----------- 40 4 TH TRANSFER ------- 10 -------- OUTCOME 20 ----------- 40 10 -----------------

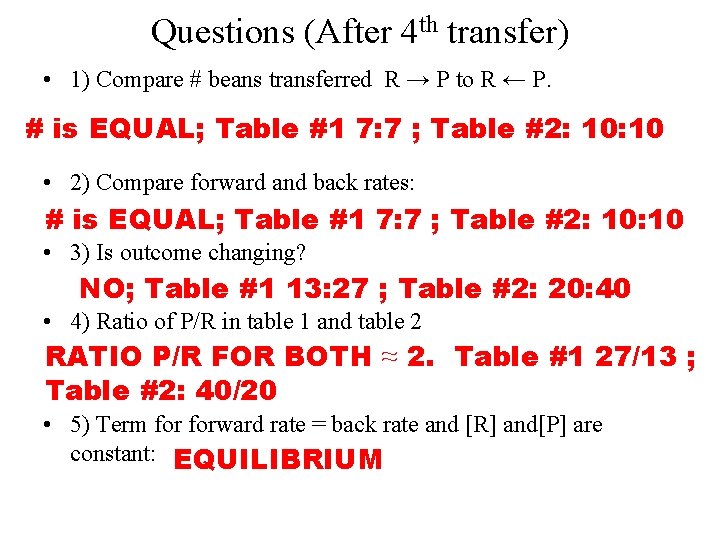
Questions (After 4 th transfer) • 1) Compare # beans transferred R → P to R ← P. # is EQUAL; Table #1 7: 7 ; Table #2: 10 • 2) Compare forward and back rates: # is EQUAL; Table #1 7: 7 ; Table #2: 10 • 3) Is outcome changing? NO; Table #1 13: 27 ; Table #2: 20: 40 • 4) Ratio of P/R in table 1 and table 2 RATIO P/R FOR BOTH ≈ 2. Table #1 27/13 ; Table #2: 40/20 • 5) Term forward rate = back rate and [R] and[P] are constant: EQUILIBRIUM