How do we know about atoms Atomic Theory

How do we know about atoms? Atomic Theory

Democritus (460 BC- 370 BC) �His mentor Leucippus �Everything is composed of “atoms” �Atoms are physically indivisible �Between atoms is empty space �Atoms are always in motion �Infinite number of atoms and kinds of atoms which differ in shape and size

John Dalton (1766 -1844) �Postulated the following: � that all matter was made of some type of particlecalled atoms which could not be broken up or destroyed �Atoms could combine with different atoms to form compounds �All atoms of the same element are the same size (mass) and have the same properties �Atoms can be rearranged during a chemical reaction to form new substances
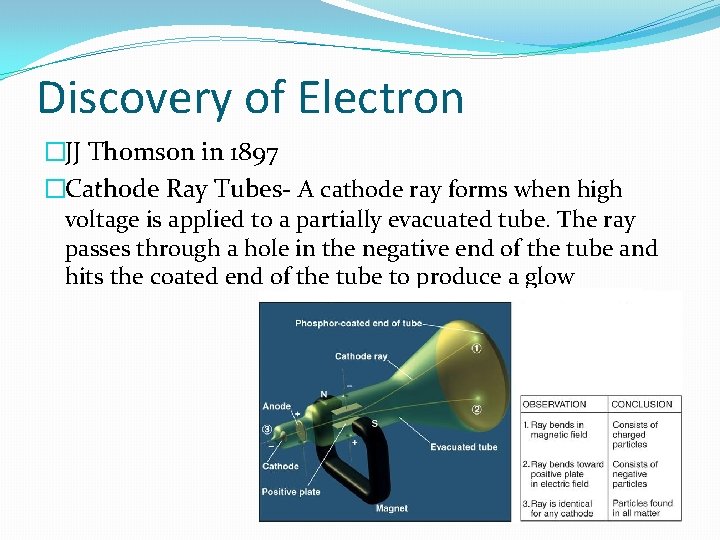
Discovery of Electron �JJ Thomson in 1897 �Cathode Ray Tubes- A cathode ray forms when high voltage is applied to a partially evacuated tube. The ray passes through a hole in the negative end of the tube and hits the coated end of the tube to produce a glow

Plum Pudding Model �Know that there are electrons (negative) �Must be a positive part as well in order to be neutral
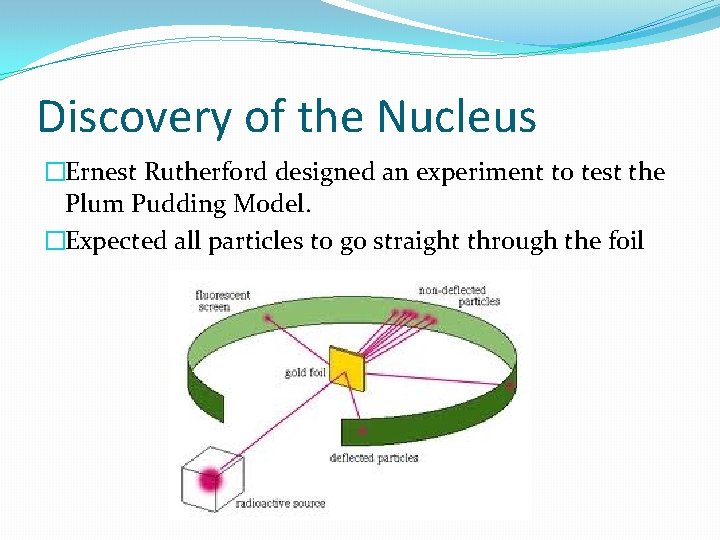
Discovery of the Nucleus �Ernest Rutherford designed an experiment to test the Plum Pudding Model. �Expected all particles to go straight through the foil

�They didn’t all go straight through? �Why?
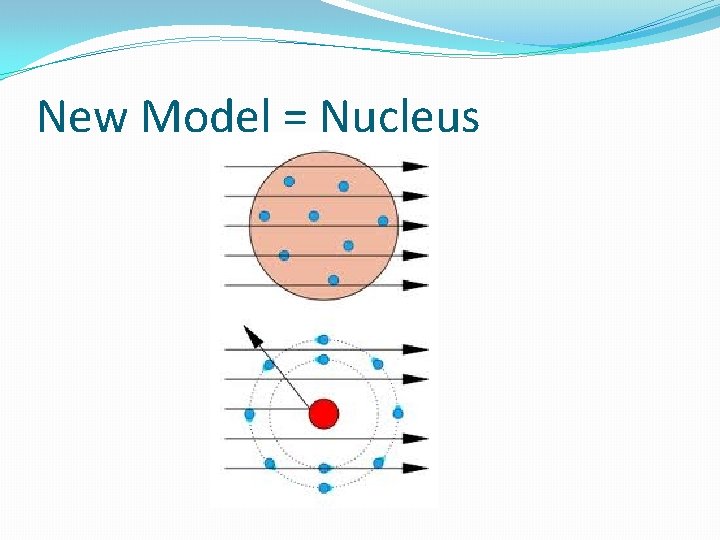
New Model = Nucleus
Nucleus �Contains the mass of the atom. � 20 years later, James Chadwick discovered the neutron because the entire mass of the nucleus could not be accounted for by just protons.

Re-examining Dalton’s Atomic Theory �Which postulates had to be modified after JJ Thomson and Ernest Rutherford’s experiments?
�All atoms are the same size- isotopes �Atoms can’t be changed- nuclear reactions �All mater is made of atoms – a proton is still matter
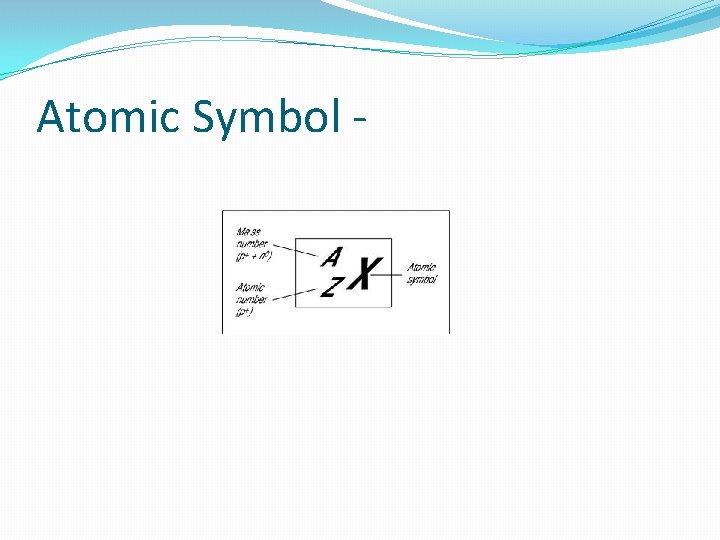
Atomic Symbol -
Isotopes �All atoms of an element are identical in atomic number, but not in atomic mass. �Isotopes = different number of neutrons. �Electrons determine chemical properties of an element. �Can determine neutrons from atomic mass and atomic number

Using the info on the PT �How many protons, neutrons and electrons does an atom of iron have?

Atomic Masses on the PT �How come they have decimals? �Does a single neutron or proton weight exactly 1 amu?
Atomic Mass Unit (amu) �The mass of an atom is measured relative to the mass of an atomic standard. �The standard = carbon-12 atom. � 1 carbon-12 atom has exactly 12 amu
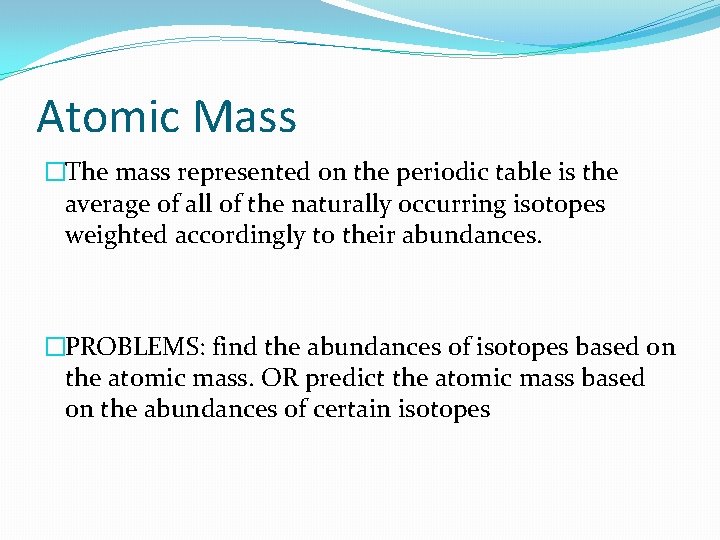
Atomic Mass �The mass represented on the periodic table is the average of all of the naturally occurring isotopes weighted accordingly to their abundances. �PROBLEMS: find the abundances of isotopes based on the atomic mass. OR predict the atomic mass based on the abundances of certain isotopes
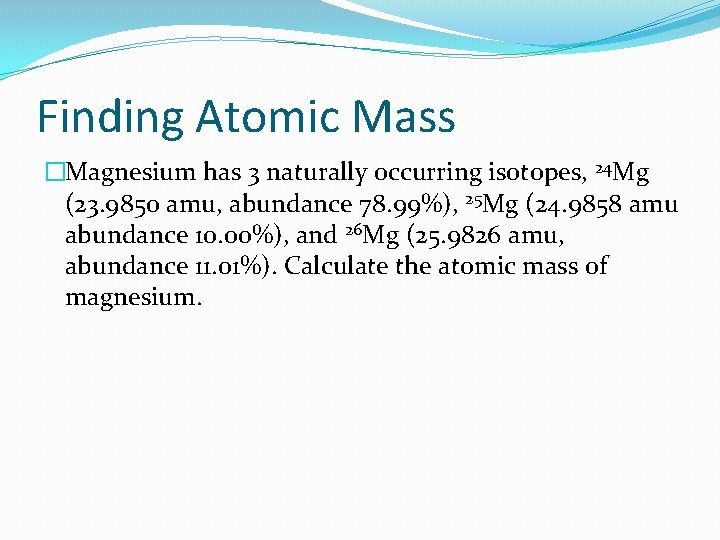
Finding Atomic Mass �Magnesium has 3 naturally occurring isotopes, 24 Mg (23. 9850 amu, abundance 78. 99%), 25 Mg (24. 9858 amu abundance 10. 00%), and 26 Mg (25. 9826 amu, abundance 11. 01%). Calculate the atomic mass of magnesium.

Sample Problem �Boron has 2 naturally occurring isotopes. Find the percent abundance of Boron-10 and Boron-11 given the atomic mass = 10. 81 amu. The isotopic mass of Boron 10 = 10. 0129 amu, and the isotopic mass of Boron-11 = 11. 093 amu.

Homework: �Pg 69: # 1 (a, b, c), 2, 9, 20 (a, b, c) 21, 22, 23 (a, b), 29, 32, 91 �Read about mass spectroscopy on page 48, and summarize what it is
- Slides: 20