How Do Guns Work How do they produce













- Slides: 21

How Do Guns Work? How do they produce evidence?

When the trigger is pressed: • The hammer drives the firing pin into the primer • The primer ignites, which causes the propellant (gunpowder) to ignite • The explosion propels the bullet out of the shell casing through the barrel • As the bullet travels through the barrel, it spins animation

Rifling • The barrel of a gun has lands and grooves. • These cause the bullet to spin so that it will fly true • Grooves are “machined” into the barrel • Number, spacing, and direction of grooves represent class evidence


Rifling (cont’d) • Imperfections in lands and grooves cause striations (no two gun barrels are exactly alike) • Striations are caused by minute imperfections in the rifling cutter, or by small bits of metal pushed against the barrel as grooves are created • Shotguns have smooth barrels (no rifling)

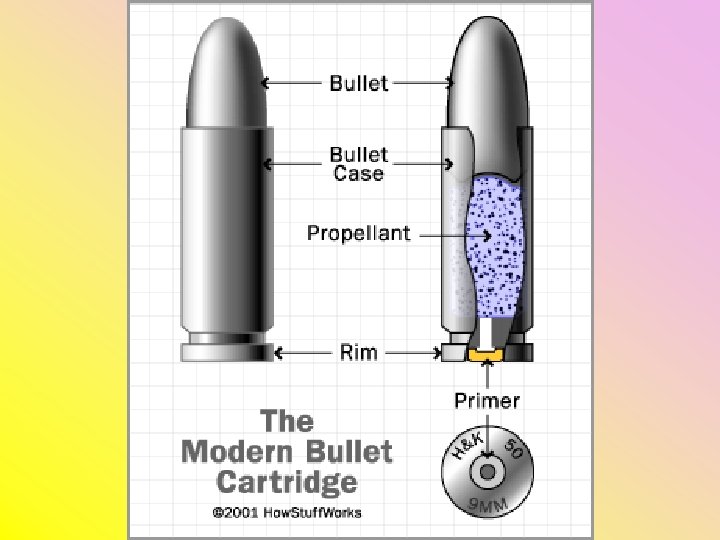
Bullet Design • A shell case contains a primer, which is ignited by the firing pin • The propellant is ignited by the primer, which causes a large explosion – The bullet is expelled at a high speed – Remnants of propellant (burned and unburned) are also expelled


Types of Evidence Created • The firing pin creates an impression on the primer in the shell casing – This impression is a toolmark, which can be matched to a single firing pin, due to irregularities • The shell is blown backwards into the breechblock, which makes marks on the shell casing

• The shell is ejected by some weapons, which creates metal-to-metal contact with the shell – These markings can be compared to test-fired shell casings • When the explosion occurs, gunshot residue (burned and unburned gunpowder) are expelled forwards and backwards. – The shooter will have residue on his/her hands and clothing


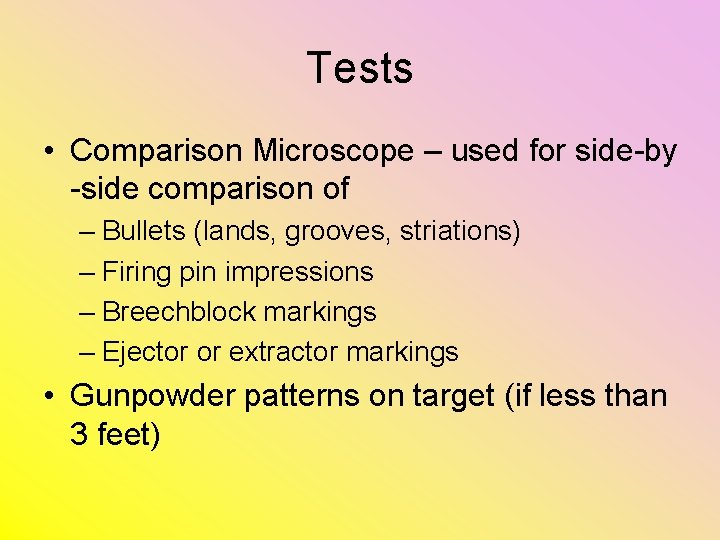
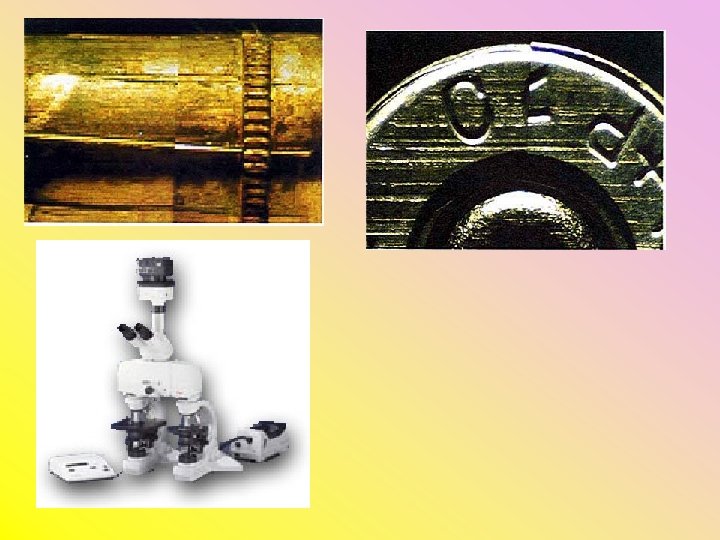
Tests • Comparison Microscope – used for side-by -side comparison of – Bullets (lands, grooves, striations) – Firing pin impressions – Breechblock markings – Ejector or extractor markings • Gunpowder patterns on target (if less than 3 feet)



• Chemical tests on powder residues – Greiss test – chemically treated photographic paper is ironed onto the target. Further chemical treatment will make nitrites visible – Lead residue test – sodium rhodizonate solution sprayed on garment, followed by acid solutions. Lead will show as pink color, followed by blue-violet color. – Test of hands for primer residues (containing Pb, Ba, Sb compounds) • Cotton swabs moistened with 5% HNO 3 (NAA and AA spectrophotometry) • Clear adhesive tape (SEM)


Neutron Activation Analysis • Every atom has three types of particles: – The nucleus contains protons and neutrons • Protons have positive charge – the number of protons in the nucleus indicates the element (i. e. carbon has 6 protons) • Neutrons have mass but no charge – Electrons “orbit” around the nucleus


• Isotopes – atoms of the same element (same number of protons) with different masses (different number of neutrons) • Most elements have both stable and unstable (radioactive) isotopes. • A sample is bombarded with neutrons, causing it to become radioactive. – Gamma radiation is measured – different elements can be identified by the energy of the gamma rays emitted. • Nondestructive • Can detect one billionth of a gram

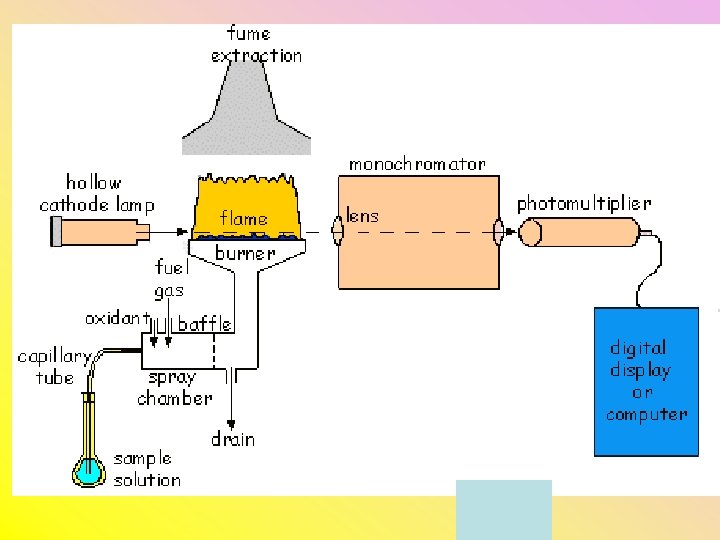
Atomic Absorption Spectroscopy • Sample is placed into air-acetylene flame to vaporize it • Gaseous sample is exposed to radiation (light) from the suspected element • The light passes through a monochromator (prism or diffraction grating) • A detector measures the radiation that is not absorbed by the sample


• Only good to detect one element at a time • Can quantitatively detect element • Can detect one trillionth of a gram with “flameless” AA (uses a furnace or hot piece of metal)