How can we determine the order of reactivity



- Slides: 4

How can we determine the order of reactivity? Baseline (Flightpath D): List the order of common metals in the reactivity series. Use general equations to write specific word equations for metals listed in the reactivity series reacting with oxygen, water, and acid. Safely make and record observations. Further (Flightpath C&B); Describe oxidation and reduction in terms of gain or loss of oxygen. Write word equations for the metals listed in the reactivity series reacting with oxygen, water, and acid, and balance given symbol equations. Predict observations for the metals listed in the reactivity series reacting with oxygen, water, and acid. Challenge Flightpath A): Justify uses of metals in the reactivity series based on their chemical reactivity. Write balanced symbol equations, with state symbols, for the metals listed in the reactivity series reacting with oxygen, water, and acid. Evaluate in detail the investigation of metals plus acid, assessing the control of variables and the validity of conclusions drawn from the data collected.

Complete the following general word equations: • metal + oxygen → • metal + water → • metal + acid → • metal + oxygen → metal oxide • metal + water → metal hydroxide + hydrogen • metal + acid → metal salt + hydrogen

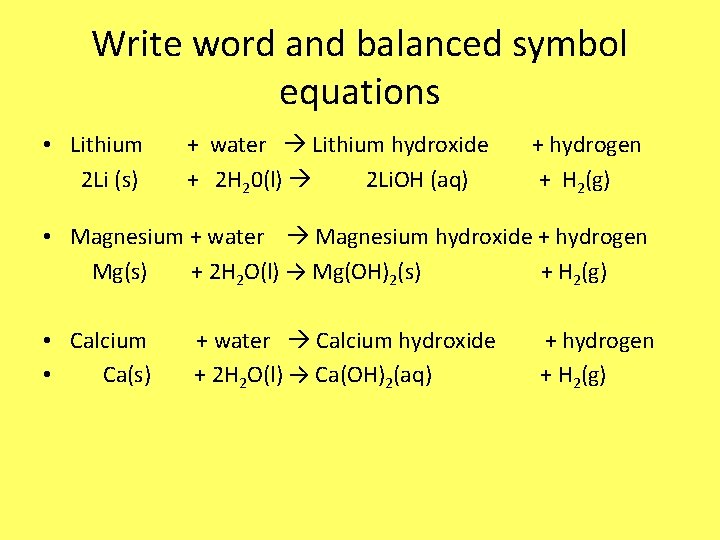
Write word and balanced symbol equations • Lithium 2 Li (s) + water Lithium hydroxide + 2 H 20(l) 2 Li. OH (aq) + hydrogen + H 2(g) • Magnesium + water Magnesium hydroxide + hydrogen Mg(s) + 2 H 2 O(l) → Mg(OH)2(s) + H 2(g) • Calcium • Ca(s) + water Calcium hydroxide + 2 H 2 O(l) → Ca(OH)2(aq) + hydrogen + H 2(g)

Metals and acids • • Add 2 cm 3 Hydrochloric acid to a test tube Add ½ spatula copper Record your observations in a table Repeat using aluminium, zinc, iron and magnesium