How can we determine the limiting reagent in
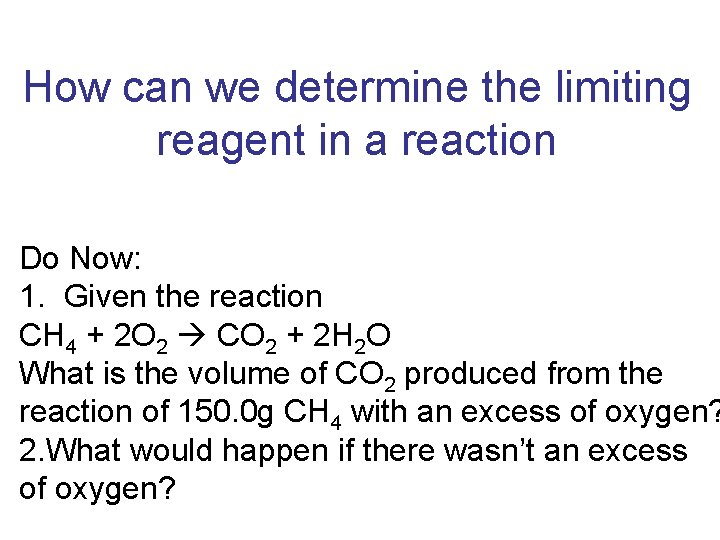







- Slides: 12
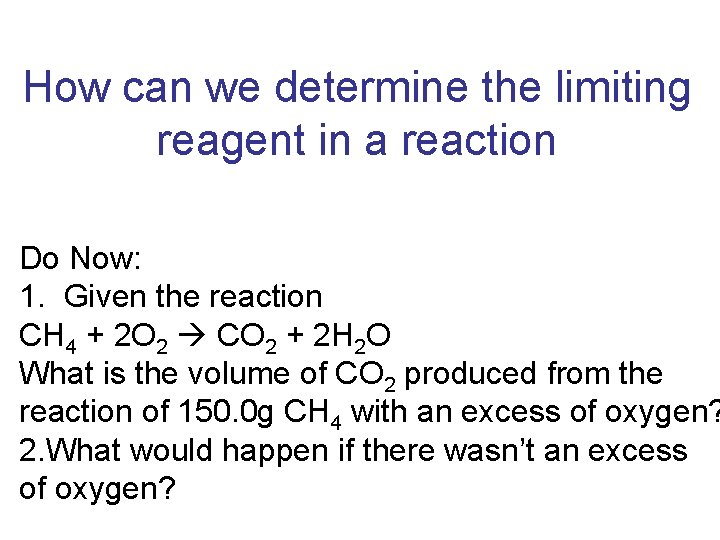
How can we determine the limiting reagent in a reaction Do Now: 1. Given the reaction CH 4 + 2 O 2 CO 2 + 2 H 2 O What is the volume of CO 2 produced from the reaction of 150. 0 g CH 4 with an excess of oxygen? 2. What would happen if there wasn’t an excess of oxygen?

I think I need a sandwich…

Turkey Sandwich • • 2 slices of bread 2 slice of turkey 1 Slice of tomato 2 slices of pickle • How many sandwiches can I make if I have 10 slices of bread an excess of all other ingredients? 10 X • 2 B + 2 T + 1 To + 2 P 1 S • But what if I only had 3 tomato slices? ? ? • How much bread remains?

Limiting Reagents • The limiting reagent in a reaction is the one that will limit the amount of product being formed • How can we determine the limiting reagent?

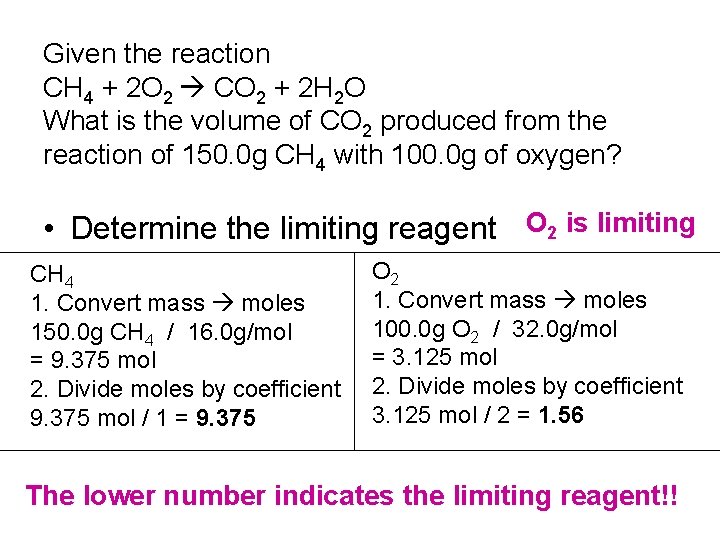
Given the reaction CH 4 + 2 O 2 CO 2 + 2 H 2 O What is the volume of CO 2 produced from the reaction of 150. 0 g CH 4 with 100. 0 g of oxygen? Determine the limiting reagent 1. Determine the number of moles of each reactant 2. Divide the number of moles by its coefficient 3. Smallest number is limiting

Given the reaction CH 4 + 2 O 2 CO 2 + 2 H 2 O What is the volume of CO 2 produced from the reaction of 150. 0 g CH 4 with 100. 0 g of oxygen? • Determine the limiting reagent O 2 is limiting CH 4 1. Convert mass moles 150. 0 g CH 4 / 16. 0 g/mol = 9. 375 mol 2. Divide moles by coefficient 9. 375 mol / 1 = 9. 375 O 2 1. Convert mass moles 100. 0 g O 2 / 32. 0 g/mol = 3. 125 mol 2. Divide moles by coefficient 3. 125 mol / 2 = 1. 56 The lower number indicates the limiting reagent!!

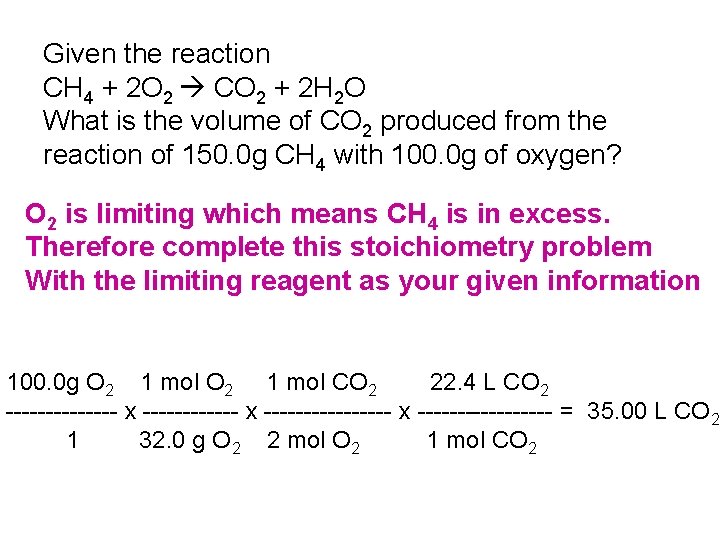
Given the reaction CH 4 + 2 O 2 CO 2 + 2 H 2 O What is the volume of CO 2 produced from the reaction of 150. 0 g CH 4 with 100. 0 g of oxygen? O 2 is limiting which means CH 4 is in excess. Therefore complete this stoichiometry problem With the limiting reagent as your given information 100. 0 g O 2 1 mol CO 2 22. 4 L CO 2 ------- x ---------------- x --------- = 35. 00 L CO 2 1 32. 0 g O 2 2 mol O 2 1 mol CO 2

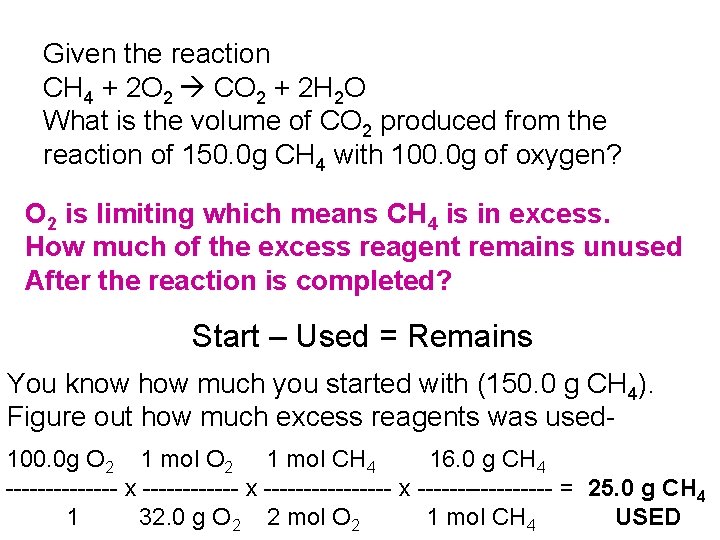
Given the reaction CH 4 + 2 O 2 CO 2 + 2 H 2 O What is the volume of CO 2 produced from the reaction of 150. 0 g CH 4 with 100. 0 g of oxygen? O 2 is limiting which means CH 4 is in excess. How much of the excess reagent remains unused After the reaction is completed? Start – Used = Remains You know how much you started with (150. 0 g CH 4). Figure out how much excess reagents was used 100. 0 g O 2 1 mol CH 4 16. 0 g CH 4 ------- x ---------------- x --------- = 25. 0 g CH 4 1 32. 0 g O 2 2 mol O 2 1 mol CH 4 USED

How much excess reagent remains? • • Start Used -------Remains 150. 0 g - 25 g ------125 g

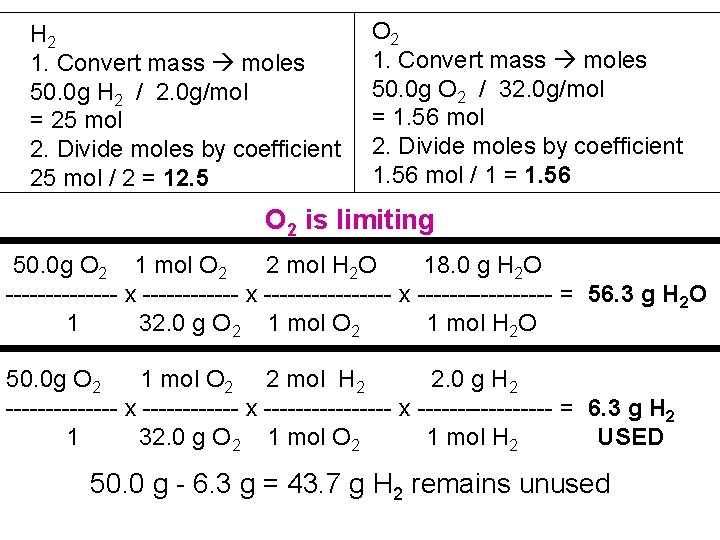
2 H 2 + O 2 2 H 2 O 1. 50. 0 g of hydrogen gas reacts with 50. 0 g of oxygen gas according to the above balanced reaction. Which reactant is the limiting reagent? 2. What mass of water is produced from this reaction? 3. What mass of the excess reactant remains unused?

H 2 1. Convert mass moles 50. 0 g H 2 / 2. 0 g/mol = 25 mol 2. Divide moles by coefficient 25 mol / 2 = 12. 5 O 2 1. Convert mass moles 50. 0 g O 2 / 32. 0 g/mol = 1. 56 mol 2. Divide moles by coefficient 1. 56 mol / 1 = 1. 56 O 2 is limiting 50. 0 g O 2 1 mol O 2 2 mol H 2 O 18. 0 g H 2 O ------- x ---------------- x --------- = 56. 3 g H 2 O 1 32. 0 g O 2 1 mol H 2 O 50. 0 g O 2 1 mol O 2 2 mol H 2 2. 0 g H 2 ------- x ---------------- x --------- = 6. 3 g H 2 1 32. 0 g O 2 1 mol H 2 USED 50. 0 g - 6. 3 g = 43. 7 g H 2 remains unused

You should be able to… • Determine the limiting reagent and the excess reagent in a reaction • Determine the amount of a product produced based on the limiting reagent • Determine the mass of excess reagent used • Determine the mass of excess reagent remaining unused