Hosted by Chem Istry Types of Reactions Electronegativity

Hosted by Chem Istry
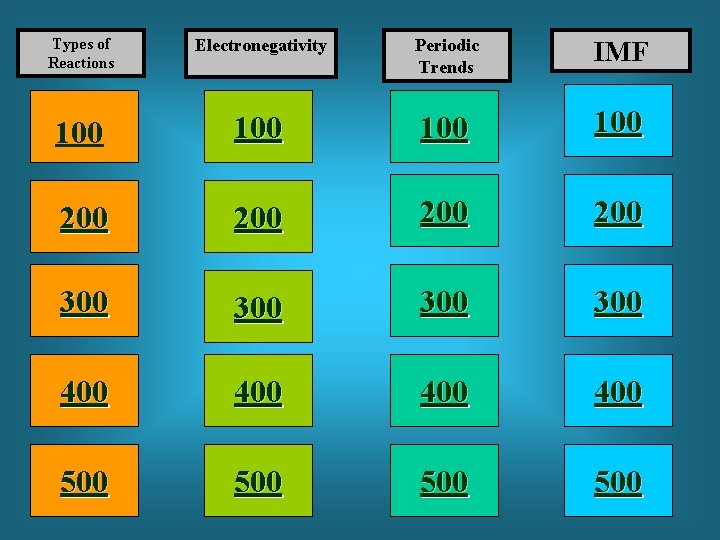
Types of Reactions Electronegativity Periodic Trends IMF 100 100 200 200 300 300 400 400 500 500
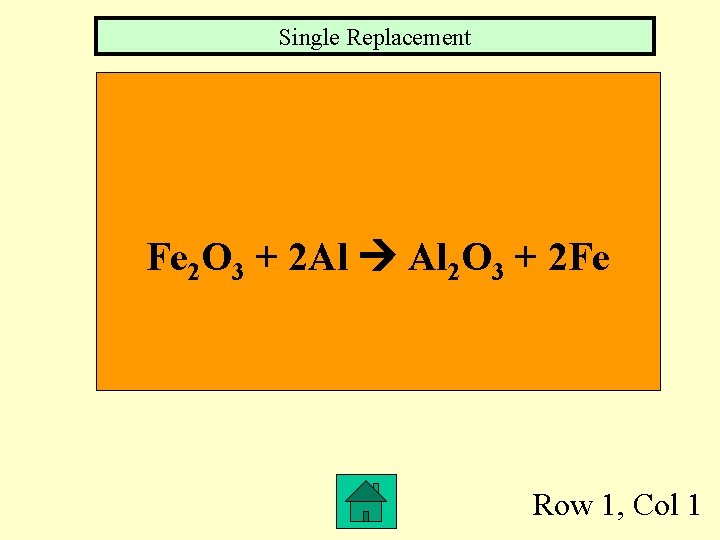
Single Replacement Fe 2 O 3 + 2 Al Al 2 O 3 + 2 Fe Row 1, Col 1

Ionic? What type of chemical bond does Mg and Br form? 1, 2

K- Potassium Which element has the largest radius? Na, Mg, K, or Ca 1, 3
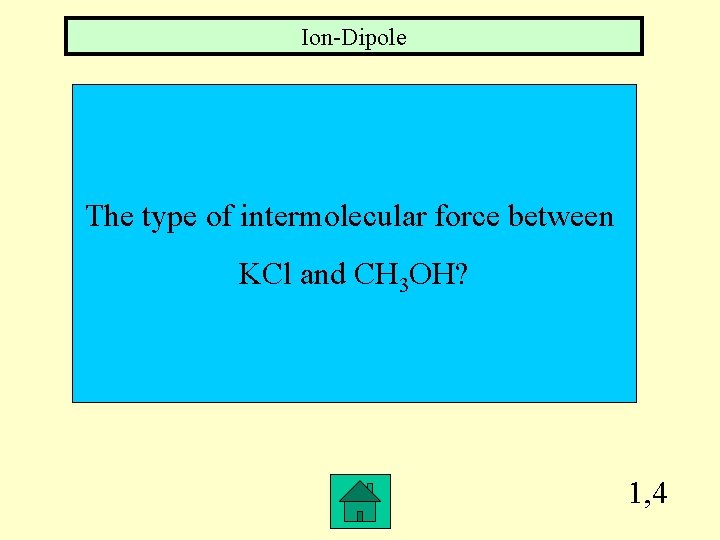
Ion-Dipole The type of intermolecular force between KCl and CH 3 OH? 1, 4
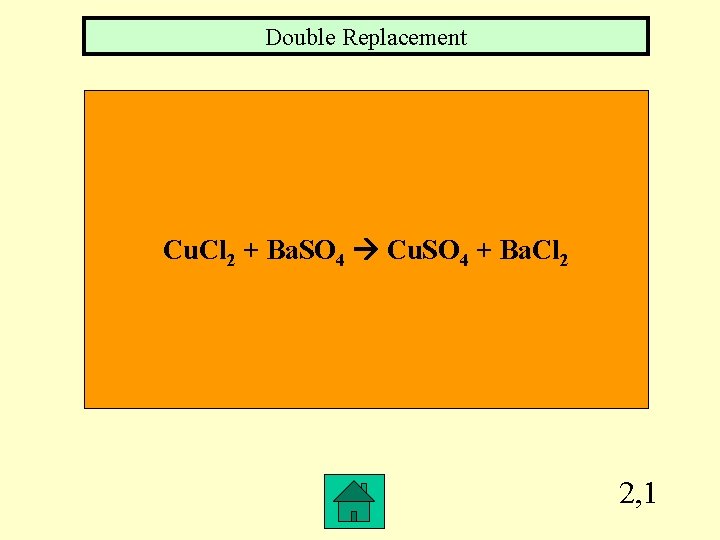
Double Replacement Cu. Cl 2 + Ba. SO 4 Cu. SO 4 + Ba. Cl 2 2, 1
Non-polar covalent Chemical bond between S and I? 2, 2

Magnesium Element with highest ionization energy. Na, Mg, K, Ca 2, 3
Dispersion (induced dipole-induced dipole) IMF between mineral oil molecules? 2, 4
Synthesis Ca. O + H 2 O Ca(OH)2 3, 1

Three NH 2 COOH (Glyceine) contains how many non-polar bonds? 3, 2
Sulfur Which element has the highest electron affinity? Se, Te, S, K, or Rb 3, 3

Mg. Cl 2 with three ions released per molecule Which substance, when added to H 2 O, will increase the boiling point the most? KCl, Mg. Cl 2, or CH 3 OH? 3, 4
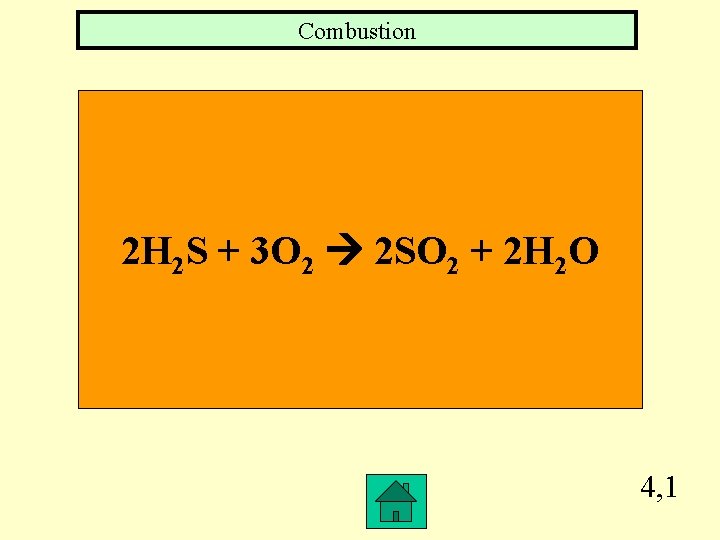
Combustion 2 H 2 S + 3 O 2 2 SO 2 + 2 H 2 O 4, 1

S-O bond at 0. 86 Which bond is more polar. C-Cl, S-O or H-Br? 4, 2

Cl-1 Which element has the smallest radius? Rb+1, Rb, Cl-1, Ca 4, 3

F 2 Which substance has the lowest boiling point? Br 2, F 2, C 2 H 6, H 2 O, Na. Cl 4, 4
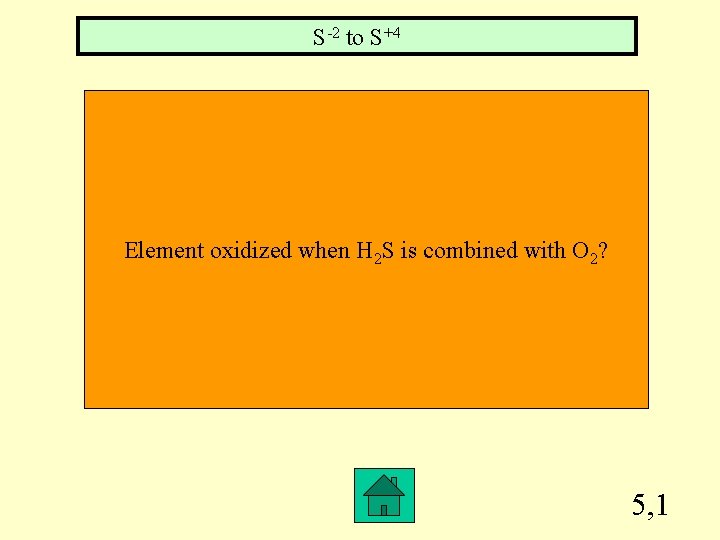
S-2 to S+4 Element oxidized when H 2 S is combined with O 2? 5, 1

Hydrogen Bonding Not a true chemical bond, but an interaction between strongly electronegative atoms of one molecule and hydrogen in another molecule. 5, 2
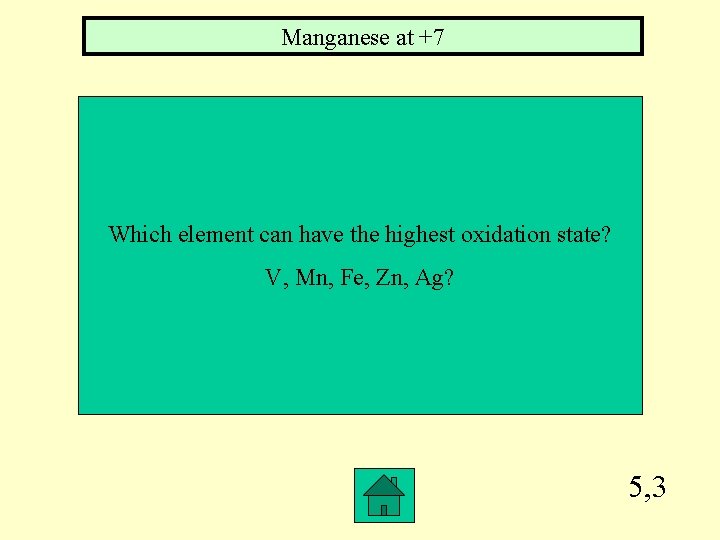
Manganese at +7 Which element can have the highest oxidation state? V, Mn, Fe, Zn, Ag? 5, 3

Mineral Oil (hydrocarbons above 20 carbons) Which non-polar substance is large enough to have a higher boiling point than water? Butane, Gasoline, Mineral Oil, Kerosene 5, 4
- Slides: 22