Honors Unit 4 Nomenclature 4 1 Naming Binary

Honors Unit 4 Nomenclature
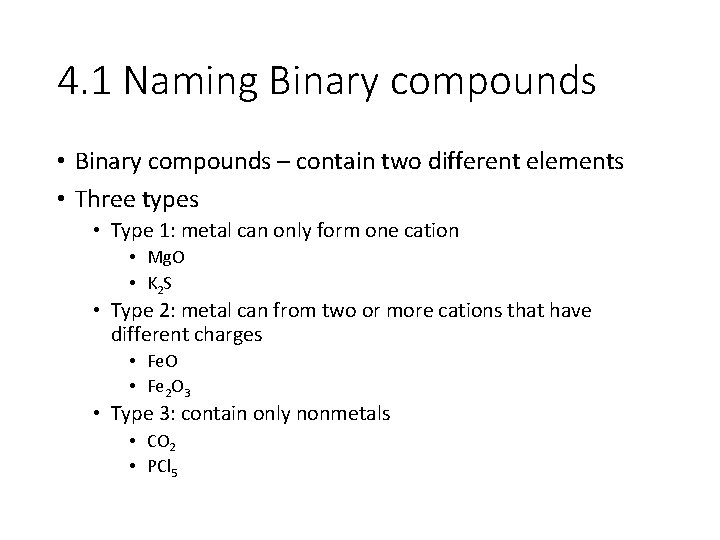
4. 1 Naming Binary compounds • Binary compounds – contain two different elements • Three types • Type 1: metal can only form one cation • Mg. O • K 2 S • Type 2: metal can from two or more cations that have different charges • Fe. O • Fe 2 O 3 • Type 3: contain only nonmetals • CO 2 • PCl 5
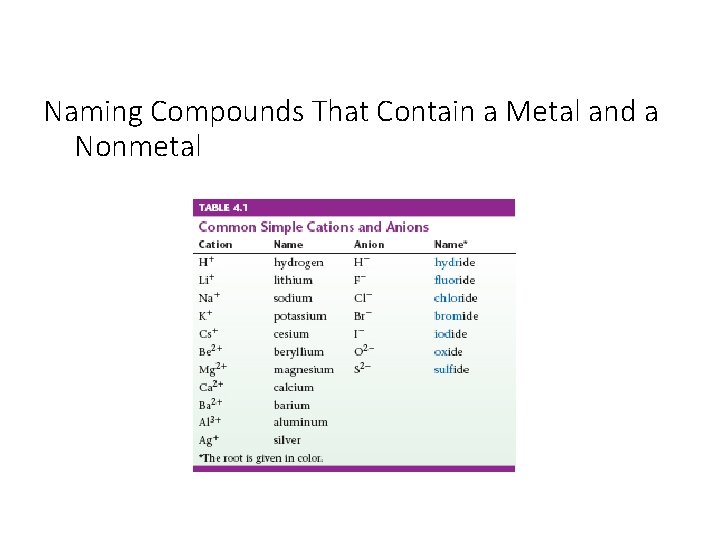
Naming Compounds That Contain a Metal and a Nonmetal

Naming Compounds That Contain a Metal and a Nonmetal Type I Binary Ionic compounds
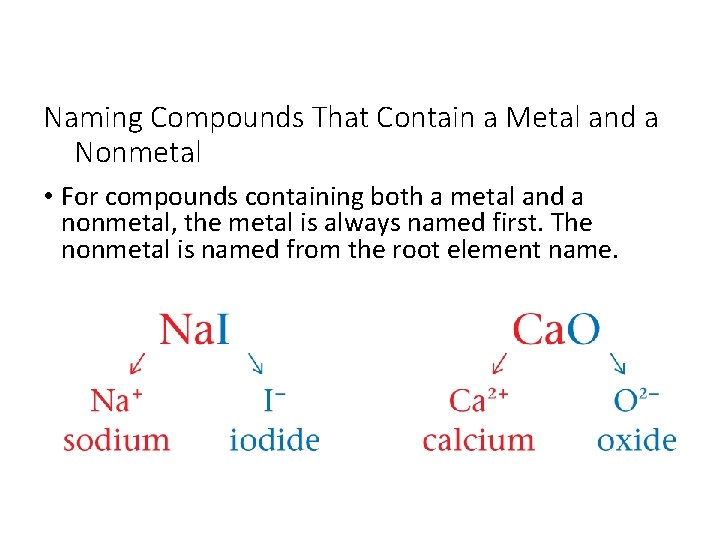
Naming Compounds That Contain a Metal and a Nonmetal • For compounds containing both a metal and a nonmetal, the metal is always named first. The nonmetal is named from the root element name.

Name the following compounds • Cs. F • Al. Cl 3 • Mg. I 2 • K 3 N

• When writing formulas of ionic compounds the net charge must be zero! • To write formulas from names: • Identify the ions involved and write their symbols • Combine the ions to create an atom with a net charge of zero

Write the formula from the name: • Calcium iodide • Sodium oxide • Aluminum nitride • Strontium phosphide
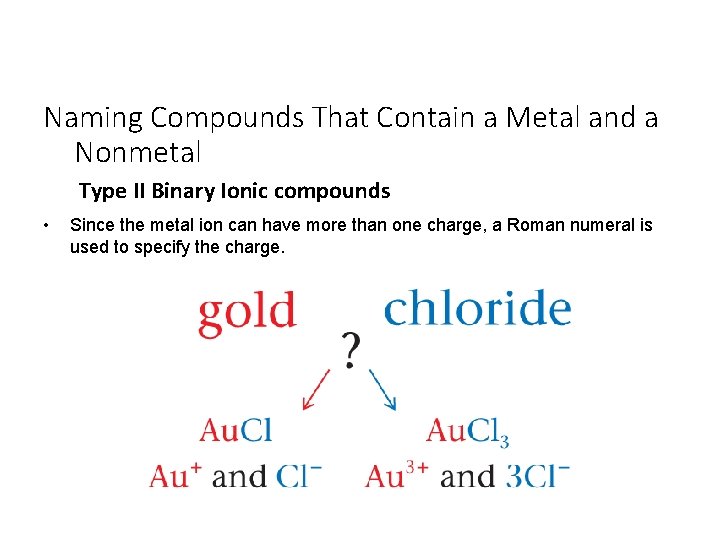
Naming Compounds That Contain a Metal and a Nonmetal Type II Binary Ionic compounds • Since the metal ion can have more than one charge, a Roman numeral is used to specify the charge.
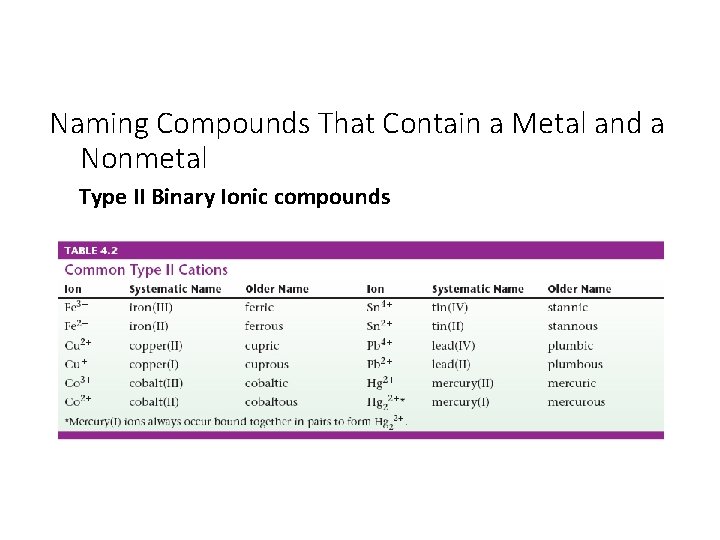
Naming Compounds That Contain a Metal and a Nonmetal Type II Binary Ionic compounds

Write the formula for the following • Iron (III) Iodide • Titanium (III) Oxide • Chromium (III) Phosphide • Copper (I) Chloride • Tin (IV) Sulfide

Name the following • Fe. Cl 2 • Pb. O 2

Name the following • Fe. S • Cu. Cl • Hg 2 O • Sn. S 2

Naming Binary Compounds That Contain Only Nonmetals (molecular compounds) Type III Compounds

Naming Binary Compounds That Contain Only Nonmetals Type III Compounds

Name The Following: • CO • H 2 O • Si 3 O 6 • P 2 Cl 5

Writing formulas from names for type III • Use the prefixes in the name to tell how many of each atom there are in the compound • DO NOT balance out charges as you do with Ionic compounds

Write the formula from the name • Carbon Tetrachloride • Sulfur Hexaflouride • Tetraphosphorus Decoxide • Dinitrogen Monoxide
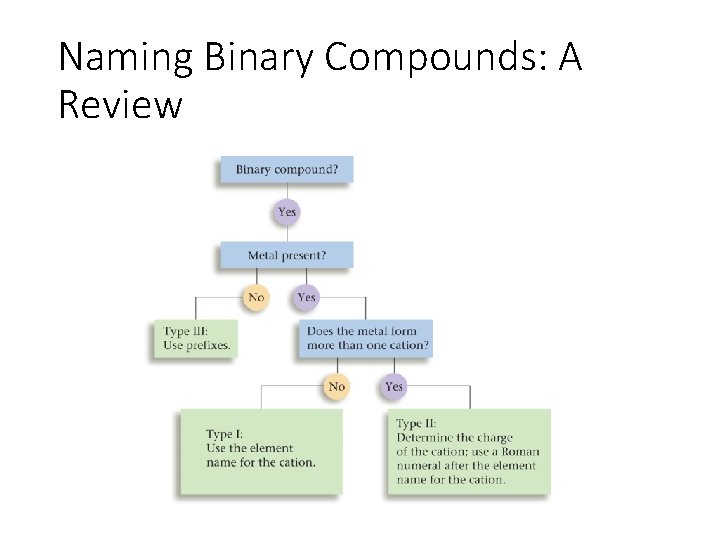
Naming Binary Compounds: A Review

Naming and Writing formulas for more Complex Compounds
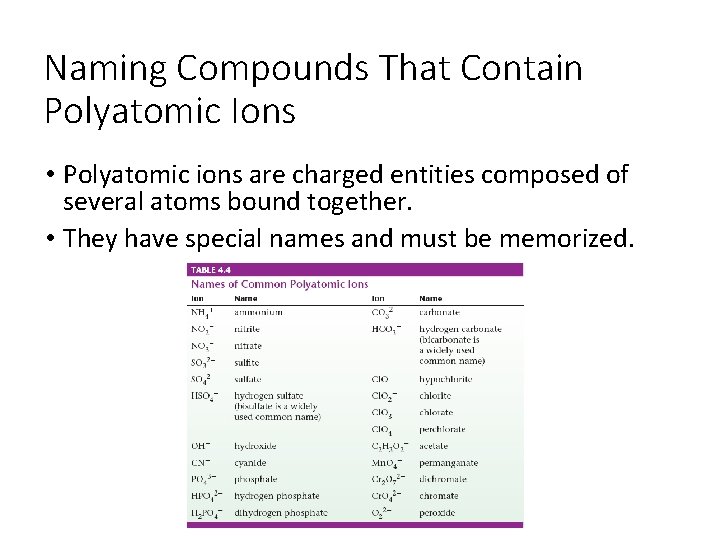
Naming Compounds That Contain Polyatomic Ions • Polyatomic ions are charged entities composed of several atoms bound together. • They have special names and must be memorized.
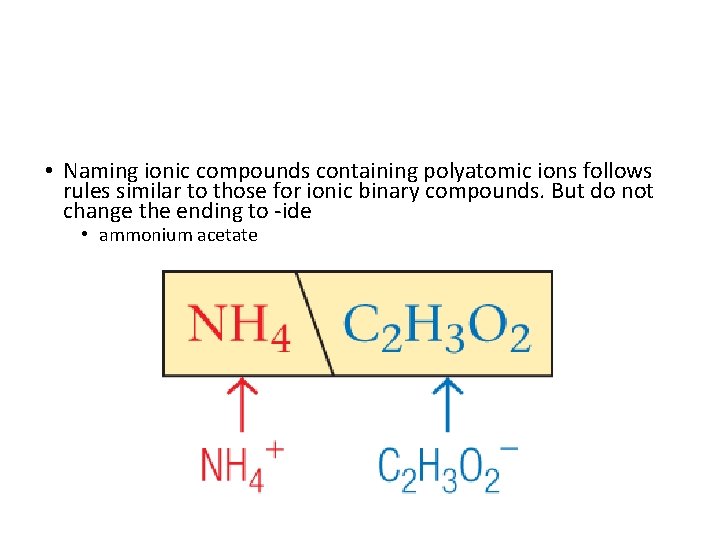
• Naming ionic compounds containing polyatomic ions follows rules similar to those for ionic binary compounds. But do not change the ending to -ide • ammonium acetate
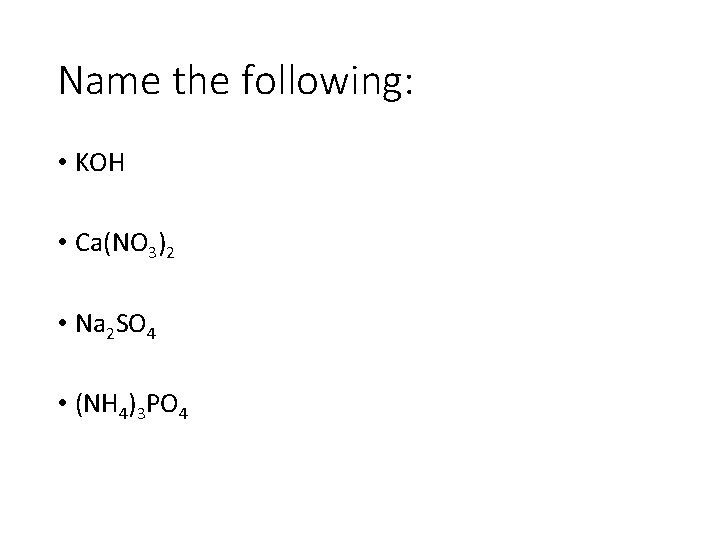
Name the following: • KOH • Ca(NO 3)2 • Na 2 SO 4 • (NH 4)3 PO 4

When writing formulas from names balance charges as you do with ionic compounds • Sodium Phosphate • Calcium Nitrate • Aluminum hydroxide • Potassium carbonate
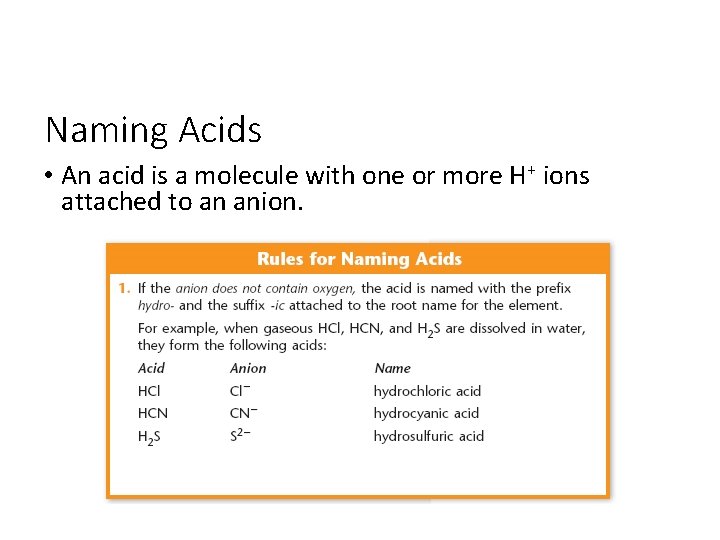
Naming Acids • An acid is a molecule with one or more H+ ions attached to an anion.
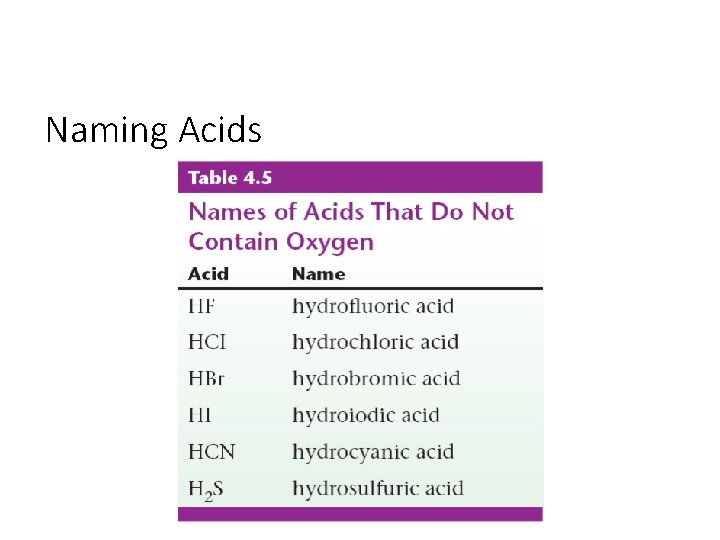
Naming Acids
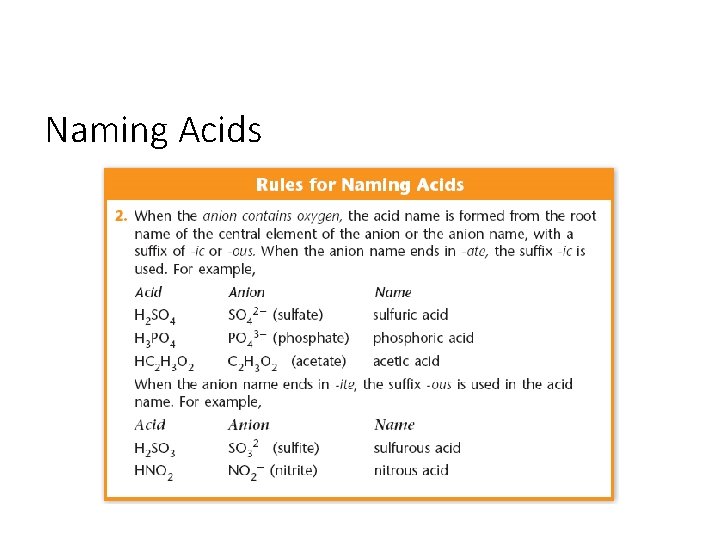
Naming Acids
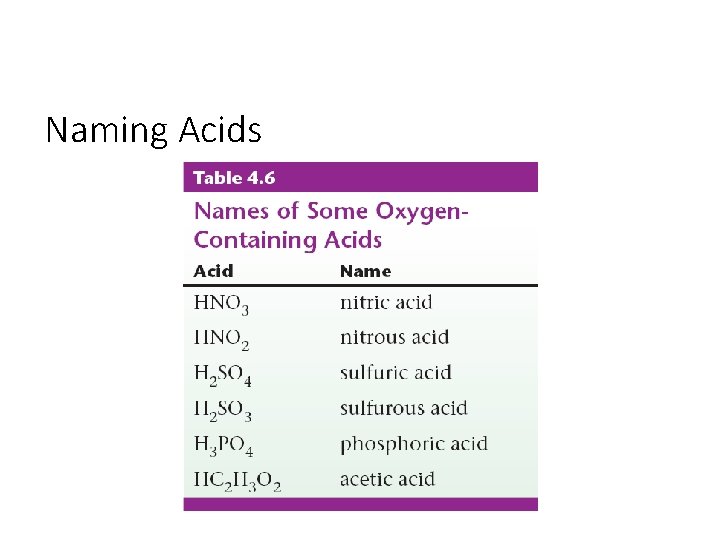
Naming Acids
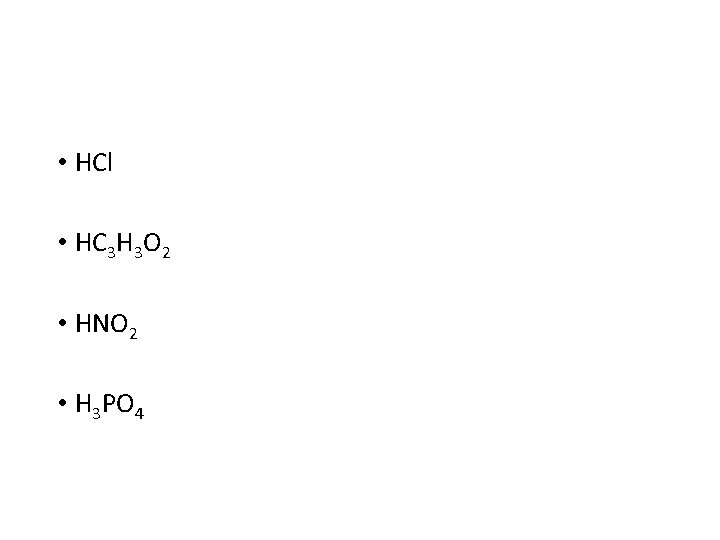
• HCl • HC 3 H 3 O 2 • HNO 2 • H 3 PO 4
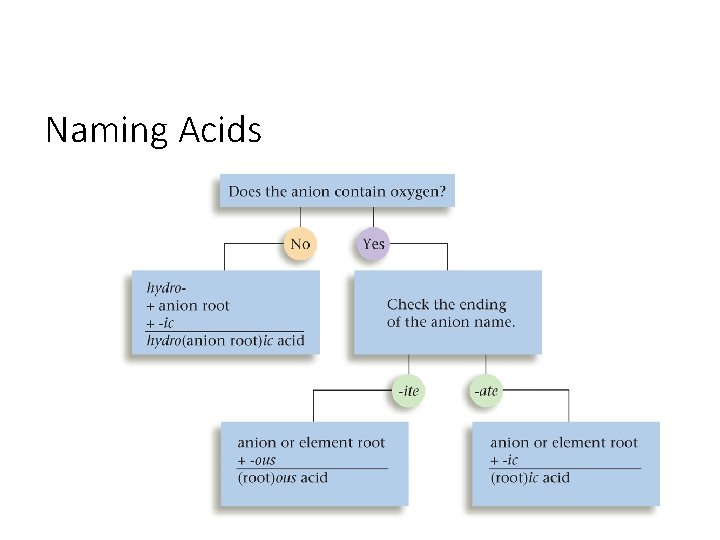
Naming Acids

Naming: All mixed up! • Na. Br • HSO 3 • Cu 2 O • P 4 O 5

Writing Formulas from Names: All mixed up! • Sodium sulfite • Magnesium carbonate • Sulfuric acid • Dinitrogen pentoxide • Cobalt(III) nitrate
- Slides: 32