Honors Physical Science TEST 2 STANDARDS OF MEASUREMENT











- Slides: 15

Honors Physical Science TEST 2: STANDARDS OF MEASUREMENT

What is a STANDARD? It is an exact quantity that people agree to use for comparison

Measurement A measurement must have a number and a unit. (No Naked Numbers!)

International System of Units SI is an improved version of the metric system and is used by scientists worldwide. It is based on multiples of ten and uses prefixes to indicate a specific multiple. Copy Table 2 “Common SI Prefixes” on p. 15 of your textbook. Copy Table 1 “SI Base Units” on p. 15 of your textbook.

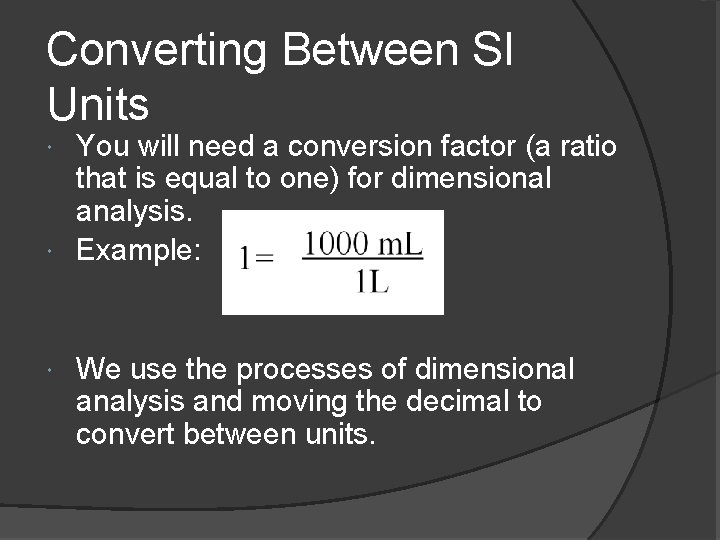
Converting Between SI Units You will need a conversion factor (a ratio that is equal to one) for dimensional analysis. Example: We use the processes of dimensional analysis and moving the decimal to convert between units.

Length Is a measure of the distance between two points Is measured using a unit appropriate for the distance between the two points SI base unit is the meter

Volume Is the amount of space an object contains The units are: liters, cubic meters (centimeters, etc. ) The volume for a solid rectangle can be measured in cubic centimeters by using the formula V=L x W x H The volume of liquids is most commonly measured in liters and milliliters. A liquid has no sides to measure so the measurement is actually the capacity of the container holding the liquid. 1 m. L = 1 cm 3 To calculate the volume of an irregular solid, we can submerge it in a known volume of liquid and read the new volume level. The volume of the object is equal to the change in volume of the liquid.

Mass Is the measure of matter in an object The most commonly used units for mass are grams and kilograms. Remember the difference between mass and weight!

Density Is the mass per unit volume of a material; D = m/v It is a derived unit (a unit obtained by combining different SI units) Density can be used to identify a substance. The most common units for density are g/m. L and g/cm 3. The density of water is 1 g/m. L. (If an object floats in water its density is less than 1 g/m. L. What do you think the density will be if an object sinks in water? )

Time Is the interval between two events The SI unit for time is the second. You may need to convert a time to seconds when solving problems. Always read the question carefully!

Temperature Is a measure of the average kinetic energy of all the particles in an object For scientific work we will use the Celsius or the Kelvin scale. To convert from Celsius to Kelvin add 273 to the Celsius reading. To convert from Kelvin to Celsius, subtract 273 from the Kelvin reading.

Practice! Measurement worksheets: Metrics and Measurement Unit Conversions & Factor Label Method Using Correct Units Scientific Notation Density

Formative Assessment Activity Floating Logs Probe Activity

SI Olympics Using SI measurements compete with your classmates to win the SI Olympics

Review for Test 2 will cover measurement. A portion of this test will take place in the lab using equipment to check your ability to make precise and accurate measurements.