Honors Biology Chapter 6 Cellular Respiration How Cells
Honors Biology Chapter 6 Cellular Respiration How Cells Harvest Chemical Energy
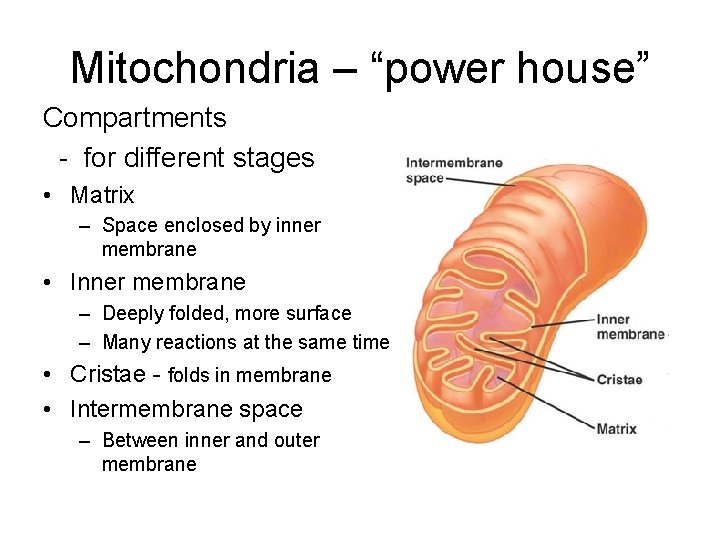
Mitochondria – “power house” Compartments - for different stages • Matrix – Space enclosed by inner membrane • Inner membrane – Deeply folded, more surface – Many reactions at the same time • Cristae - folds in membrane • Intermembrane space – Between inner and outer membrane
Honors Bio Ch. 6: Cell Respiration All life activities need energy a. Maintain homeostasis; do life functions breathe, circulate blood active transport, biosynthesis regulate temperature, etc. b. Physical and mental activity c. Cells use energy in ATP molecules

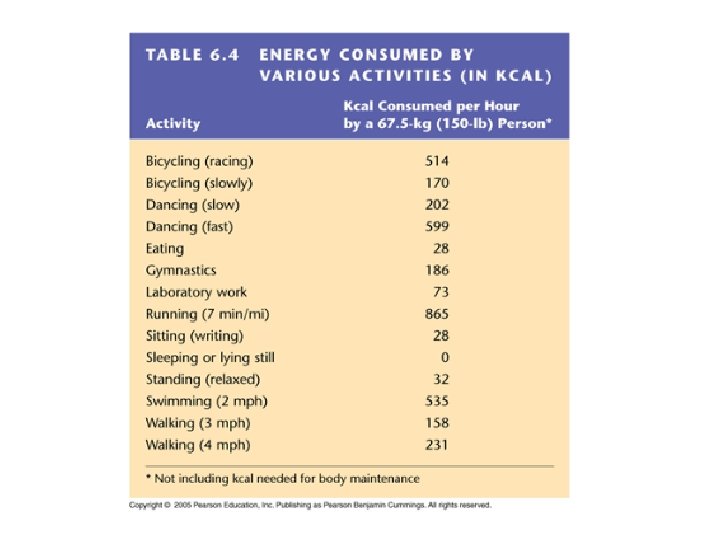
Food energy is measured in calories Food labels: Calorie (Kcal) = 1000 calories calorie = energy needed to raise the temperature of one m. L water 1 degree Celsius 1 gram carb = 4 cal 1 gram fat = 9 cal 1 gram protein = 4 cal
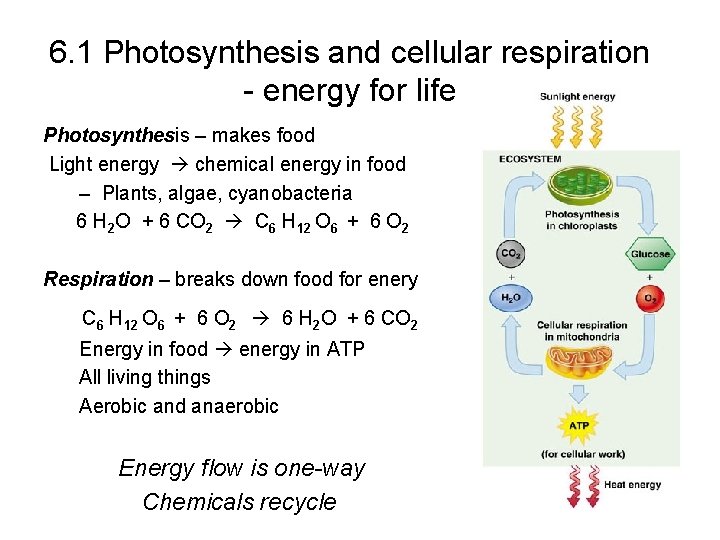
6. 1 Photosynthesis and cellular respiration - energy for life Photosynthesis – makes food Light energy chemical energy in food – Plants, algae, cyanobacteria 6 H 2 O + 6 CO 2 C 6 H 12 O 6 + 6 O 2 Respiration – breaks down food for enery C 6 H 12 O 6 + 6 O 2 6 H 2 O + 6 CO 2 Energy in food energy in ATP All living things Aerobic and anaerobic Energy flow is one-way Chemicals recycle
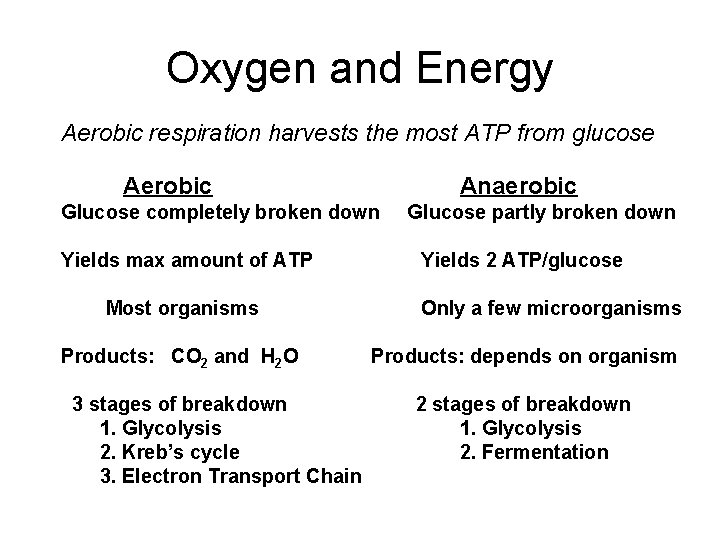
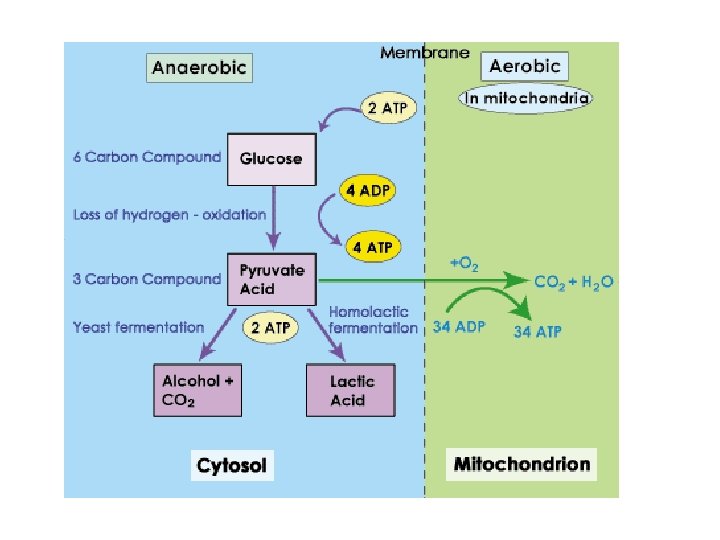
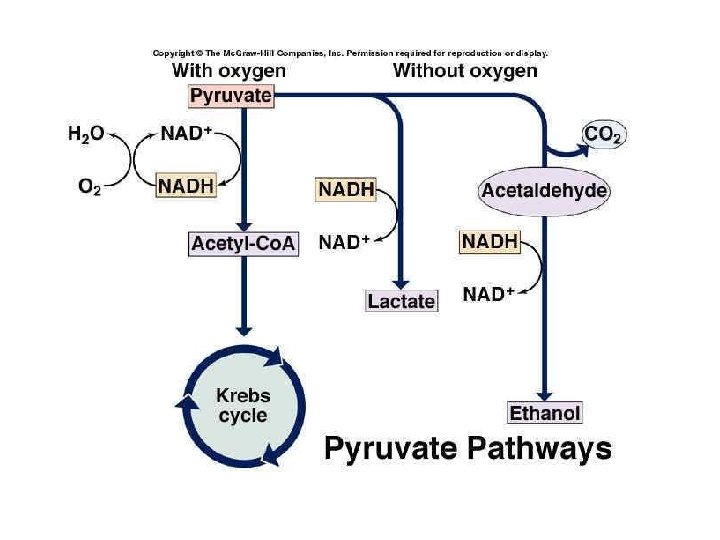
Oxygen and Energy Aerobic respiration harvests the most ATP from glucose Aerobic Anaerobic Glucose completely broken down Yields max amount of ATP Most organisms Products: CO 2 and H 2 O 3 stages of breakdown 1. Glycolysis 2. Kreb’s cycle 3. Electron Transport Chain Glucose partly broken down Yields 2 ATP/glucose Only a few microorganisms Products: depends on organism 2 stages of breakdown 1. Glycolysis 2. Fermentation
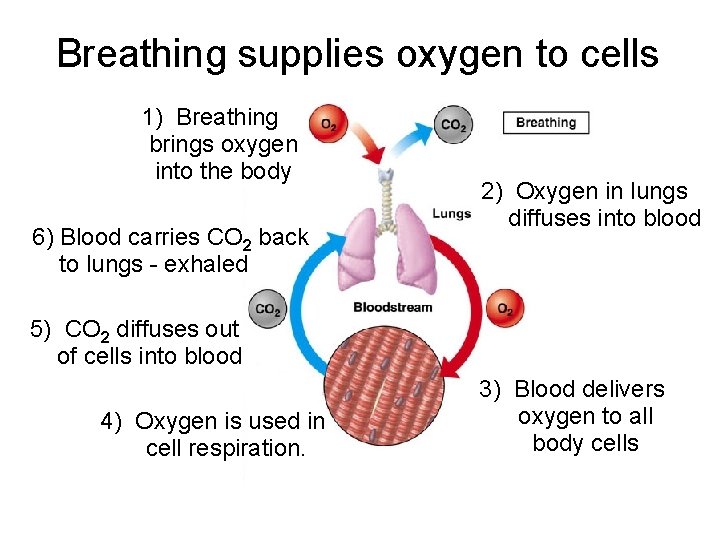
Breathing supplies oxygen to cells 1) Breathing brings oxygen into the body 6) Blood carries CO 2 back to lungs - exhaled 2) Oxygen in lungs diffuses into blood 5) CO 2 diffuses out of cells into blood 4) Oxygen is used in cell respiration. 3) Blood delivers oxygen to all body cells
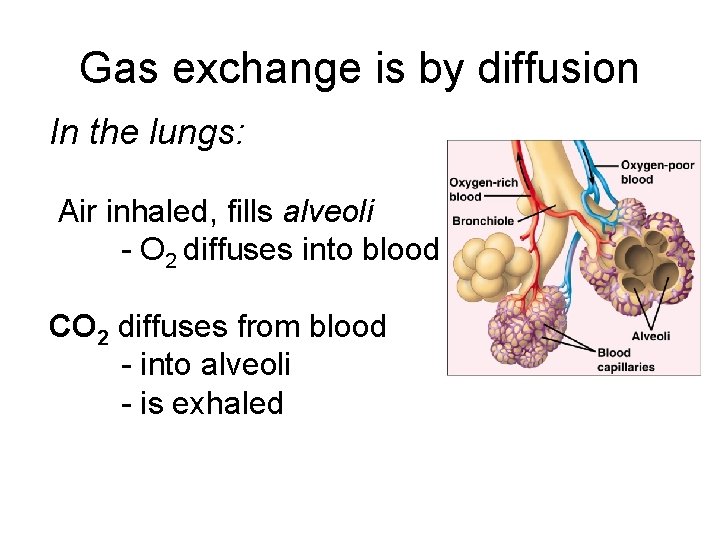
Gas exchange is by diffusion In the lungs: Air inhaled, fills alveoli - O 2 diffuses into blood CO 2 diffuses from blood - into alveoli - is exhaled
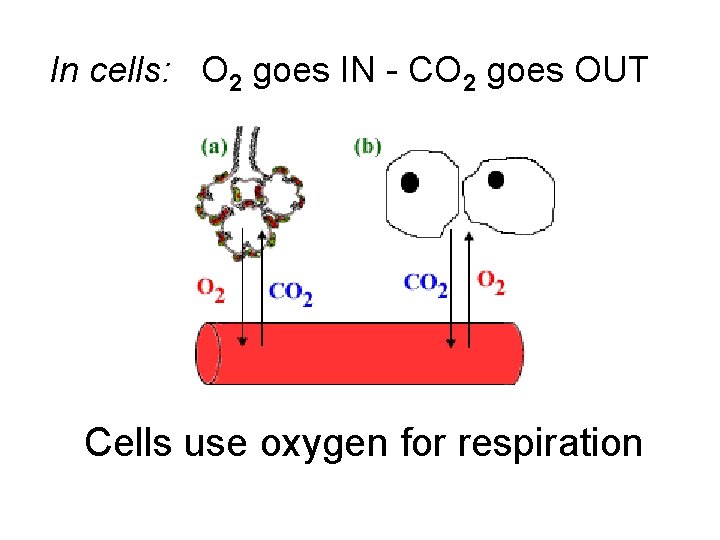
In cells: O 2 goes IN - CO 2 goes OUT Cells use oxygen for respiration
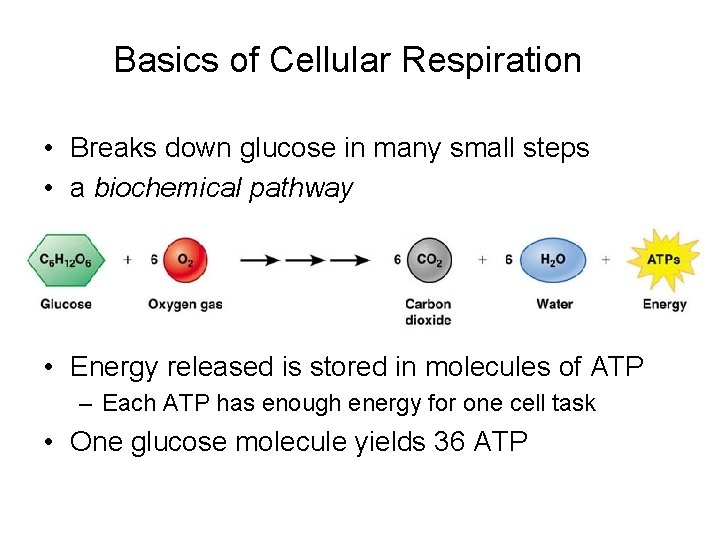
Basics of Cellular Respiration • Breaks down glucose in many small steps • a biochemical pathway • Energy released is stored in molecules of ATP – Each ATP has enough energy for one cell task • One glucose molecule yields 36 ATP
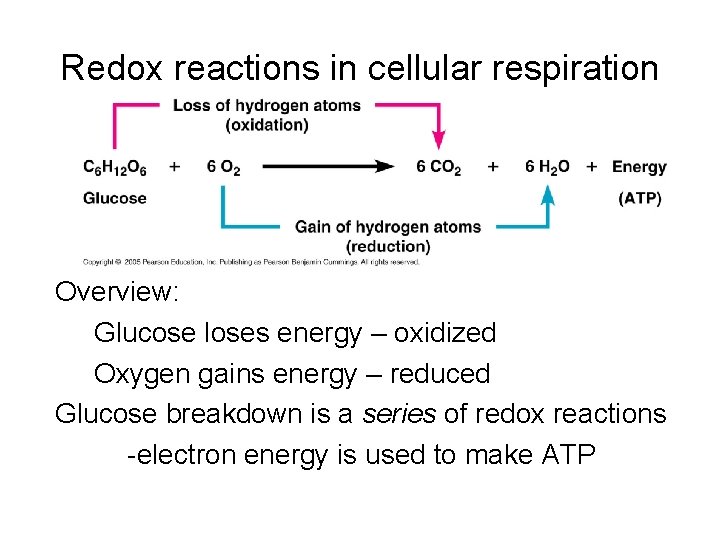
Redox reactions in cellular respiration Overview: Glucose loses energy – oxidized Oxygen gains energy – reduced Glucose breakdown is a series of redox reactions -electron energy is used to make ATP

Electron/H+ Acceptors • Help in reaction pathway, re-used • 2 in respiration: NAD and FAD • Accept hydrogen ions and electrons from glucose as it breaks down • Transfer them to another molecule later in pathway –makes ATP
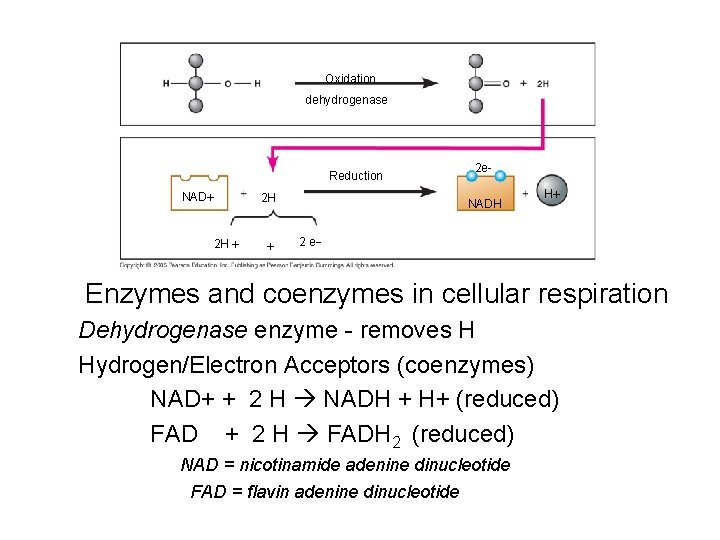
Oxidation dehydrogenase Reduction NAD 2 H 2 H 2 e. NADH H 2 e Enzymes and coenzymes in cellular respiration Dehydrogenase enzyme - removes H Hydrogen/Electron Acceptors (coenzymes) NAD+ + 2 H NADH + H+ (reduced) FAD + 2 H FADH 2 (reduced) NAD = nicotinamide adenine dinucleotide FAD = flavin adenine dinucleotide
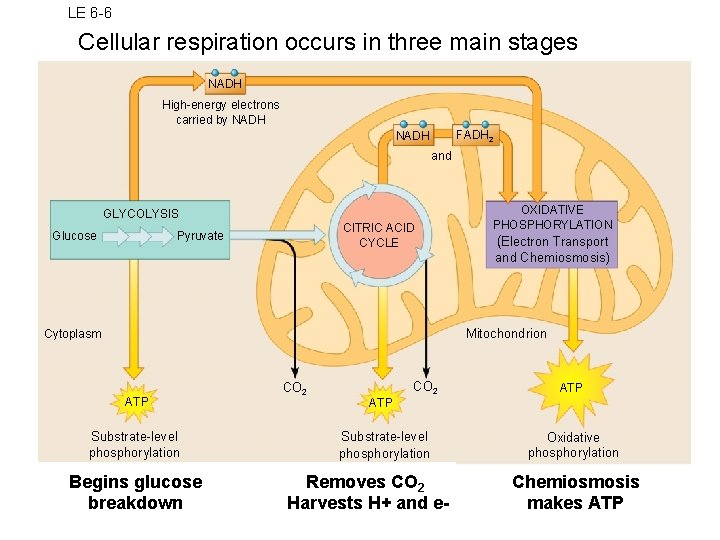
LE 6 -6 Cellular respiration occurs in three main stages NADH High-energy electrons carried by NADH FADH 2 NADH and GLYCOLYSIS Glucose CITRIC ACID CYCLE Pyruvate OXIDATIVE PHOSPHORYLATION (Electron Transport and Chemiosmosis) Mitochondrion Cytoplasm ATP Substrate-level phosphorylation Begins glucose breakdown CO 2 ATP Substrate-level phosphorylation Removes CO 2 Harvests H+ and e- Oxidative phosphorylation Chemiosmosis makes ATP
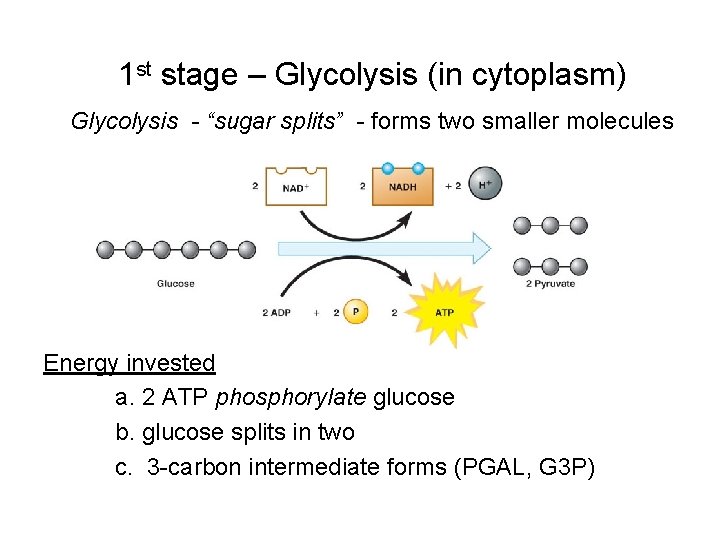
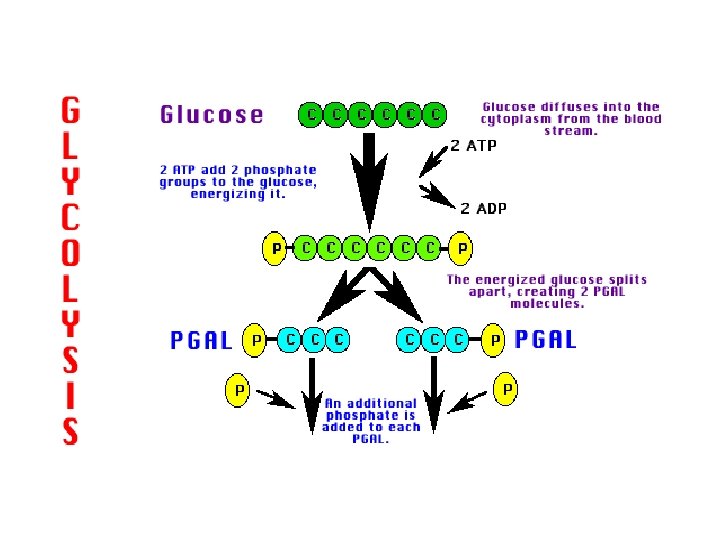
1 st stage – Glycolysis (in cytoplasm) Glycolysis - “sugar splits” - forms two smaller molecules Energy invested a. 2 ATP phosphorylate glucose b. glucose splits in two c. 3 -carbon intermediate forms (PGAL, G 3 P)
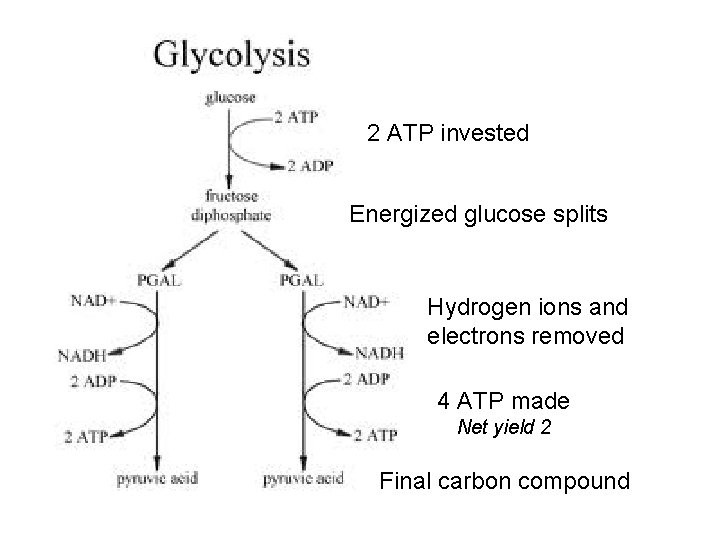
2 ATP invested Energized glucose splits Hydrogen ions and electrons removed 4 ATP made Net yield 2 Final carbon compound
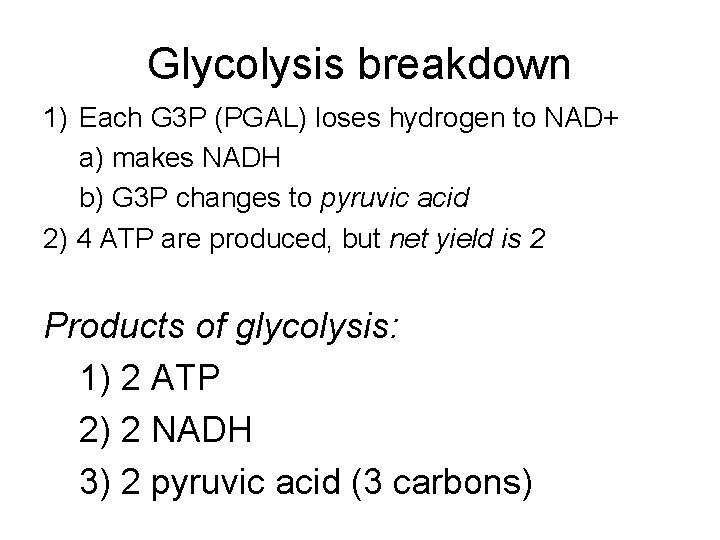
Glycolysis breakdown 1) Each G 3 P (PGAL) loses hydrogen to NAD+ a) makes NADH b) G 3 P changes to pyruvic acid 2) 4 ATP are produced, but net yield is 2 Products of glycolysis: 1) 2 ATP 2) 2 NADH 3) 2 pyruvic acid (3 carbons)

All organisms do glycolysis • Need no oxygen or special organelles • Probably evolved very early in history of life • Can meet energy needs of some simple organisms
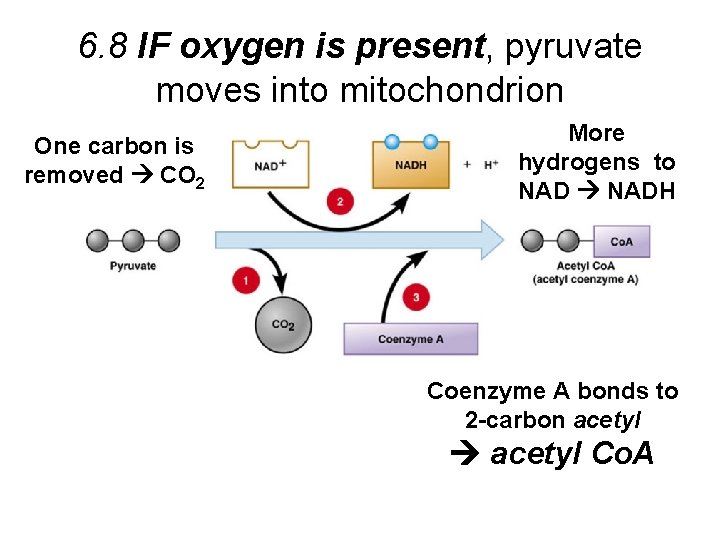
6. 8 IF oxygen is present, pyruvate moves into mitochondrion One carbon is removed CO 2 More hydrogens to NADH Coenzyme A bonds to 2 -carbon acetyl Co. A

Sir Hans Krebs 1900 -1981 • German chemist, 1930 s • Described the cycle of reactions that make energy in cells • Received Nobel in 1953 • “Krebs Cycle” or “Citric Acid Cycle”
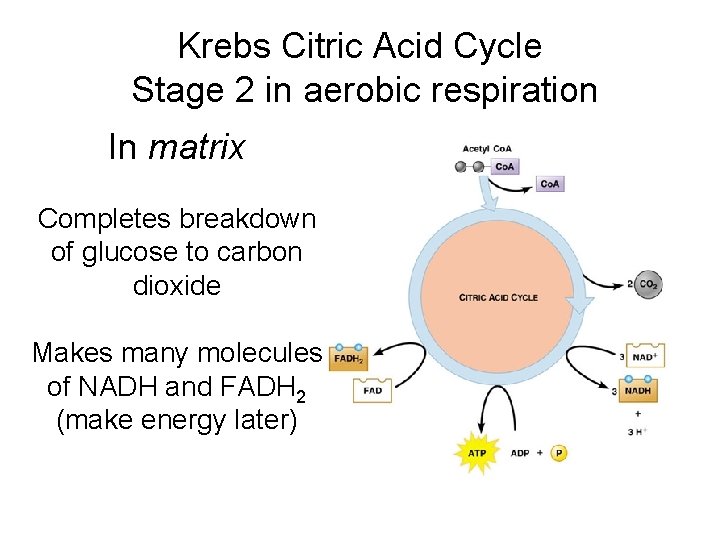
Krebs Citric Acid Cycle Stage 2 in aerobic respiration In matrix Completes breakdown of glucose to carbon dioxide Makes many molecules of NADH and FADH 2 (make energy later)
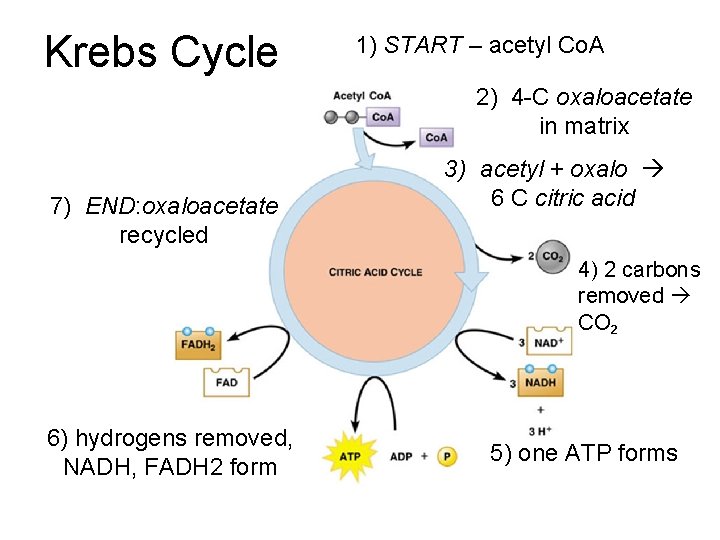
Krebs Cycle 1) START – acetyl Co. A 2) 4 -C oxaloacetate in matrix 7) END: oxaloacetate recycled 3) acetyl + oxalo 6 C citric acid 4) 2 carbons removed CO 2 6) hydrogens removed, NADH, FADH 2 form 5) one ATP forms
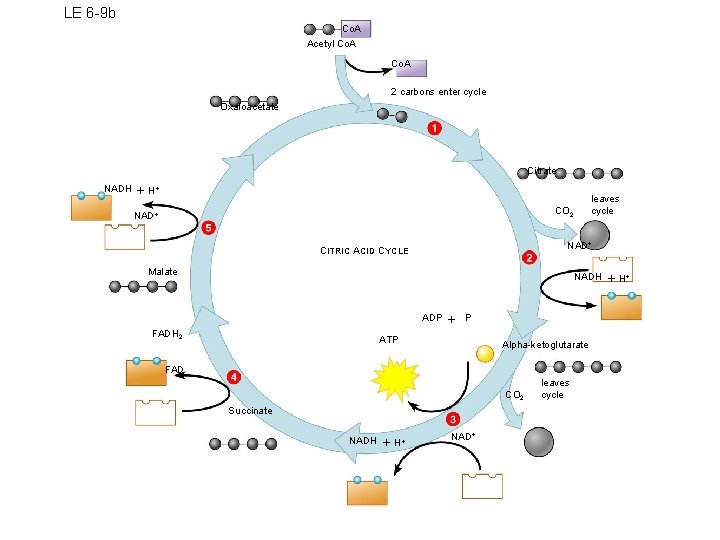
LE 6 -9 b Co. A Acetyl Co. A 2 carbons enter cycle Oxaloacetate Citrate NADH H leaves cycle CO 2 NAD CITRIC ACID CYCLE Malate NADH ADP FADH 2 P ATP Alpha-ketoglutarate FAD CO 2 Succinate NADH H NAD leaves cycle H
Products of Krebs Cycle 1. 2 ATP/glucose molecule (one each “turn”) 2. Several molecules of NADH and FADH 2 – These will yield energy in stage 3 3. Last carbons in glucose form CO 2 and diffuse out of cell
Review: Krebs Cycle 1. START – acetyl Co. A (2 C) 2. Joins 4 C compound in matrix (oxaloacetate) 3. Forms 6 C citric acid 2 CO 2 4. Carriers NAD+, FAD reduced 5. Each cycle makes 1 ATP (2 ATP/glucose) 6. 4 C compound returned 7. END: CO 2, NADH, FADH 2, ATP
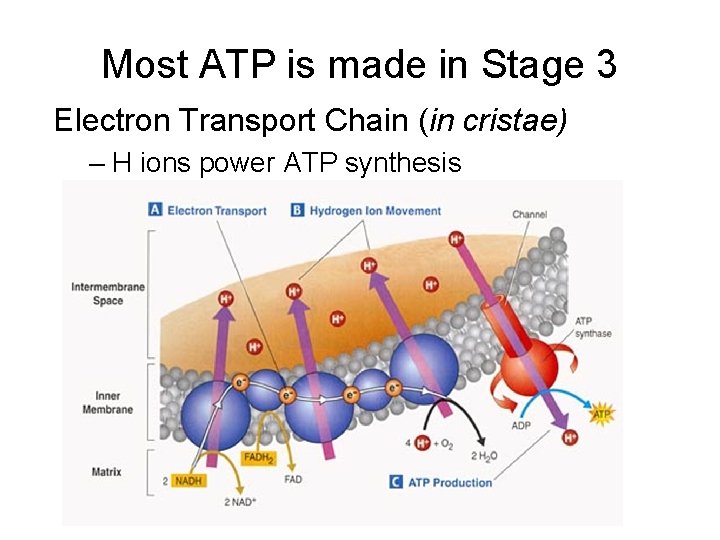
Most ATP is made in Stage 3 Electron Transport Chain (in cristae) – H ions power ATP synthesis
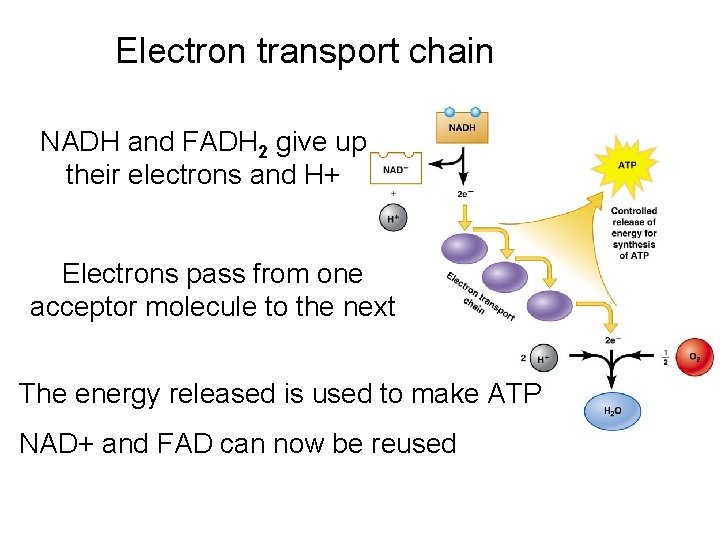
Electron transport chain NADH and FADH 2 give up their electrons and H+ Electrons pass from one acceptor molecule to the next The energy released is used to make ATP NAD+ and FAD can now be reused
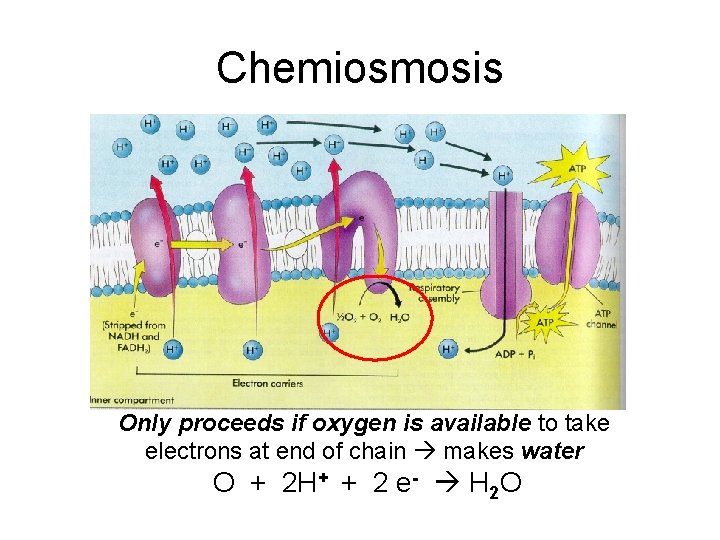
Chemiosmosis Only proceeds if oxygen is available to take electrons at end of chain makes water O + 2 H+ + 2 e- H 2 O
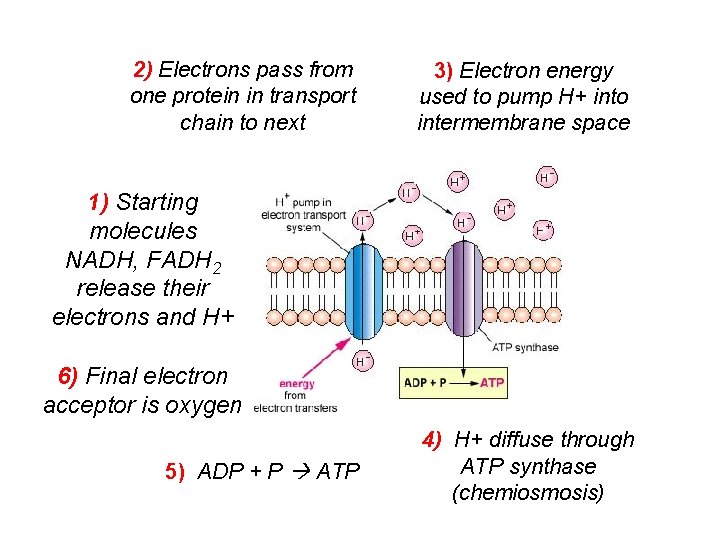
2) Electrons pass from one protein in transport chain to next 3) Electron energy used to pump H+ into intermembrane space 1) Starting molecules NADH, FADH 2 release their electrons and H+ 6) Final electron acceptor is oxygen 5) ADP + P ATP 4) H+ diffuse through ATP synthase (chemiosmosis)
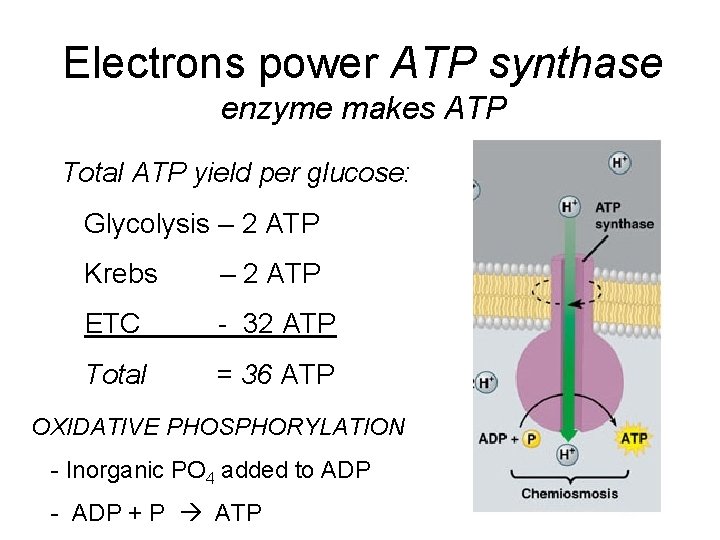
Electrons power ATP synthase enzyme makes ATP Total ATP yield per glucose: Glycolysis – 2 ATP Krebs – 2 ATP ETC - 32 ATP Total = 36 ATP OXIDATIVE PHOSPHORYLATION - Inorganic PO 4 added to ADP - ADP + P ATP
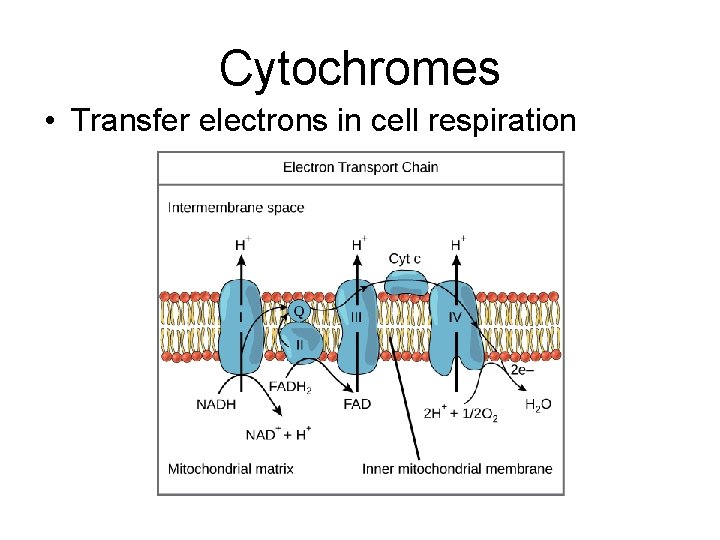
Cytochromes • Transfer electrons in cell respiration
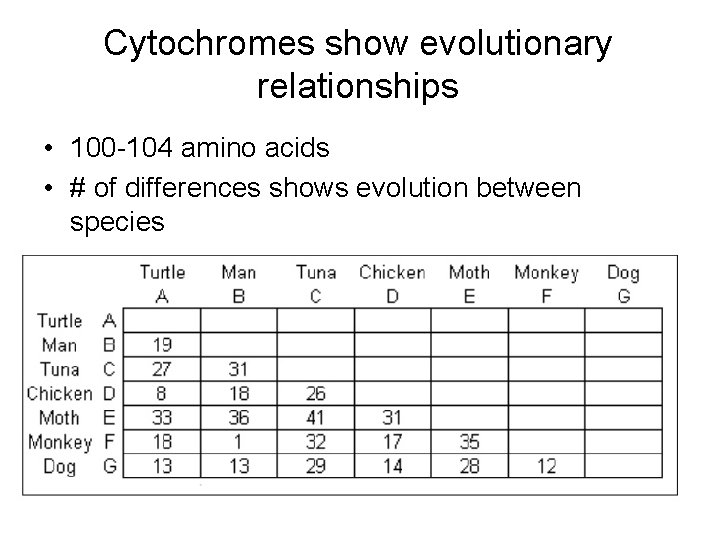
Cytochromes show evolutionary relationships • 100 -104 amino acids • # of differences shows evolution between species
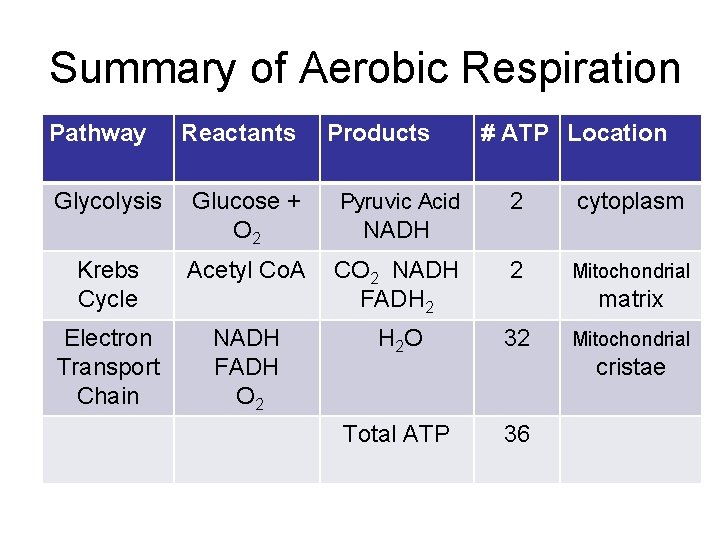
Summary of Aerobic Respiration Pathway Glycolysis Reactants Products Glucose + O 2 Pyruvic Acid Krebs Cycle Acetyl Co. A Electron Transport Chain NADH FADH O 2 # ATP Location 2 cytoplasm CO 2 NADH FADH 2 2 Mitochondrial H 2 O 32 Total ATP 36 NADH matrix Mitochondrial cristae
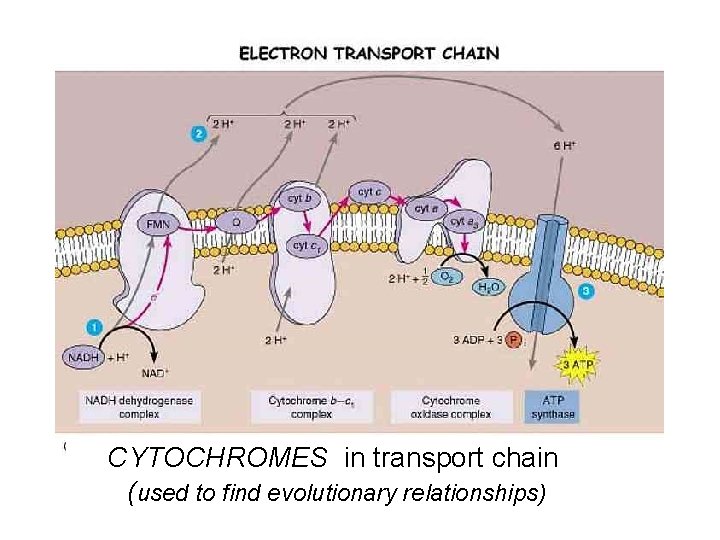
CYTOCHROMES in transport chain (used to find evolutionary relationships)
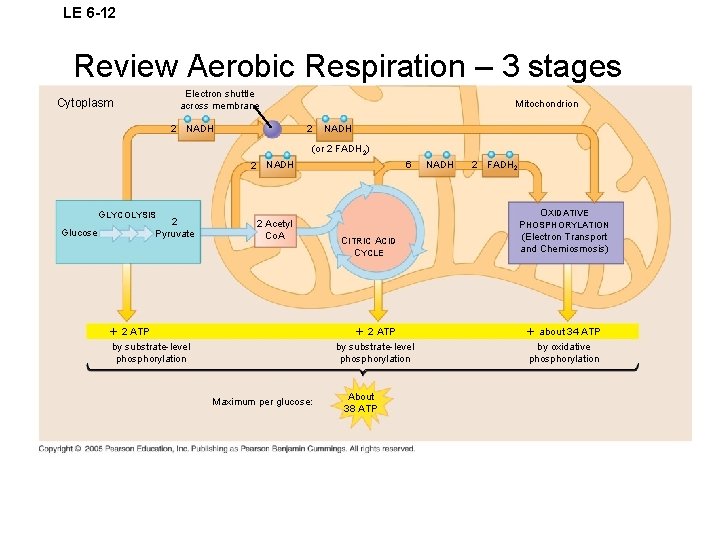
LE 6 -12 Review Aerobic Respiration – 3 stages Electron shuttle across membrane Cytoplasm 2 NADH Mitochondrion 2 NADH (or 2 FADH 2) 2 NADH GLYCOLYSIS 2 Pyruvate Glucose 2 Acetyl Co. A 2 ATP by substrate-level phosphorylation Maximum per glucose: 6 CITRIC ACID CYCLE NADH 2 FADH 2 OXIDATIVE PHOSPHORYLATION (Electron Transport and Chemiosmosis) 2 ATP about 34 ATP by substrate-level phosphorylation by oxidative phosphorylation About 38 ATP
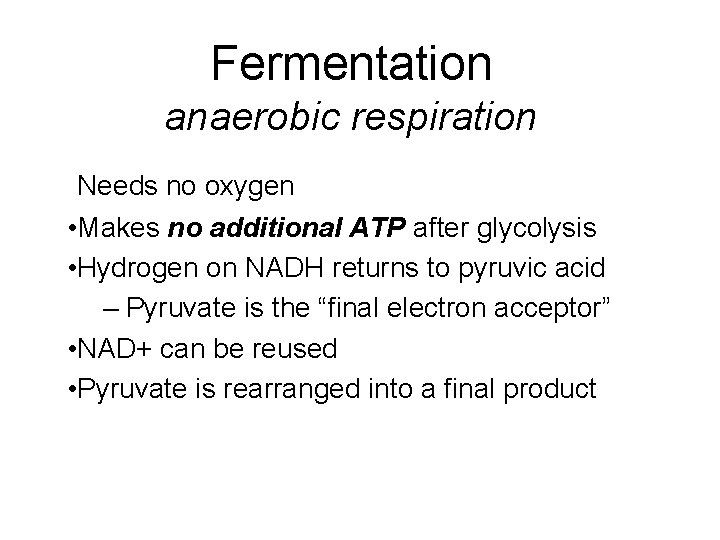
Fermentation anaerobic respiration Needs no oxygen • Makes no additional ATP after glycolysis • Hydrogen on NADH returns to pyruvic acid – Pyruvate is the “final electron acceptor” • NAD+ can be reused • Pyruvate is rearranged into a final product

Lactic Acid Fermentation • Many anaerobic bacteria • make lactic (and other) acids • Commercial uses: cheese, yogurt, soy products, sauerkraut • Muscle cells – can do fermentation temporarily • lactic acids builds up “oxygen debt” • Muscles fatigue, cramp • With fresh oxygen: Lactic acid converted back to pyruvate Kreb’s
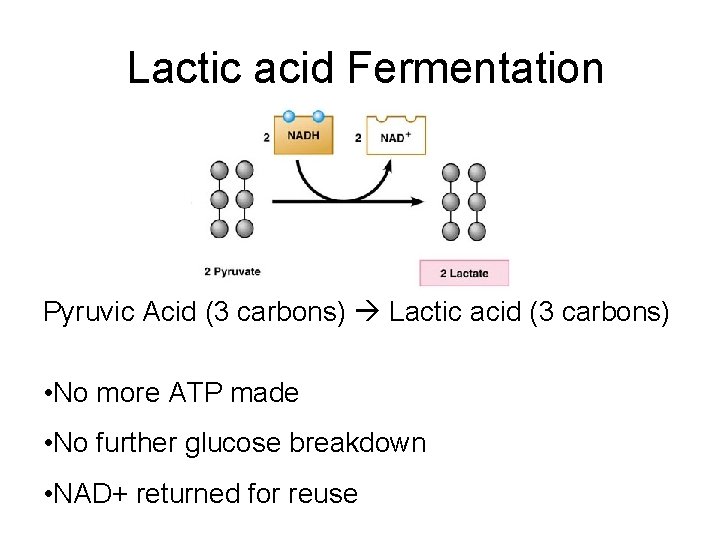
Lactic acid Fermentation Pyruvic Acid (3 carbons) Lactic acid (3 carbons) • No more ATP made • No further glucose breakdown • NAD+ returned for reuse
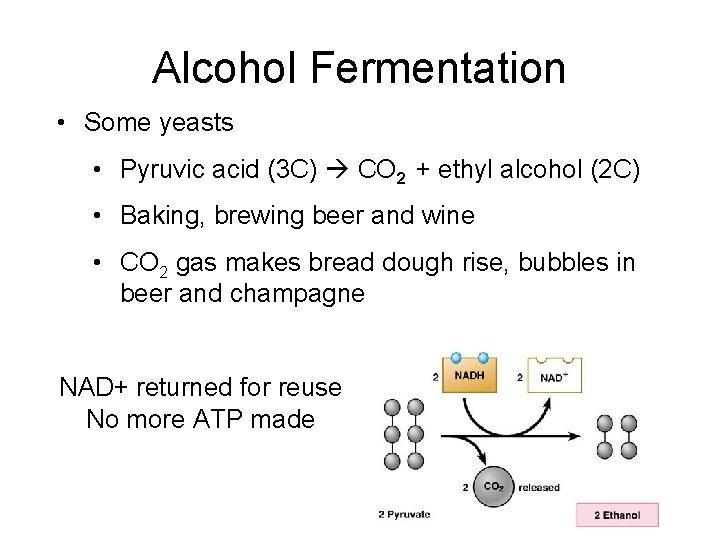
Alcohol Fermentation • Some yeasts • Pyruvic acid (3 C) CO 2 + ethyl alcohol (2 C) • Baking, brewing beer and wine • CO 2 gas makes bread dough rise, bubbles in beer and champagne NAD+ returned for reuse No more ATP made

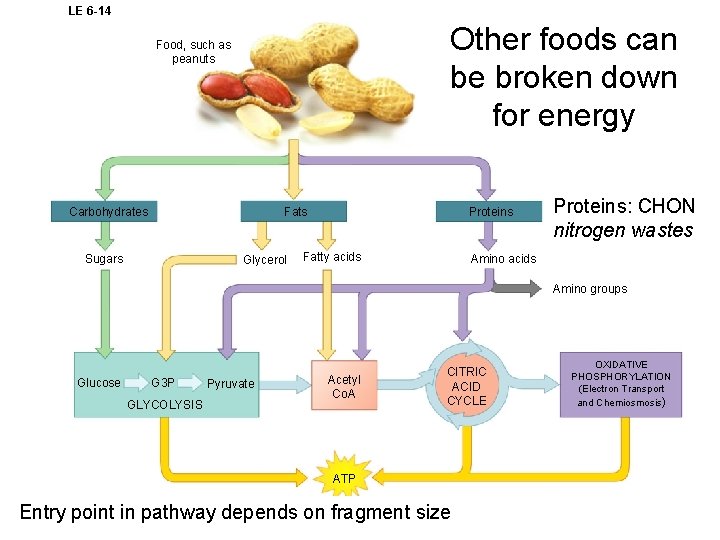
LE 6 -14 Other foods can be broken down for energy Food, such as peanuts Fats Carbohydrates Sugars Glycerol Proteins Fatty acids Proteins: CHON nitrogen wastes Amino acids Amino groups Glucose G 3 P GLYCOLYSIS Pyruvate Acetyl Co. A CITRIC ACID CYCLE ATP Entry point in pathway depends on fragment size OXIDATIVE PHOSPHORYLATION (Electron Transport and Chemiosmosis)
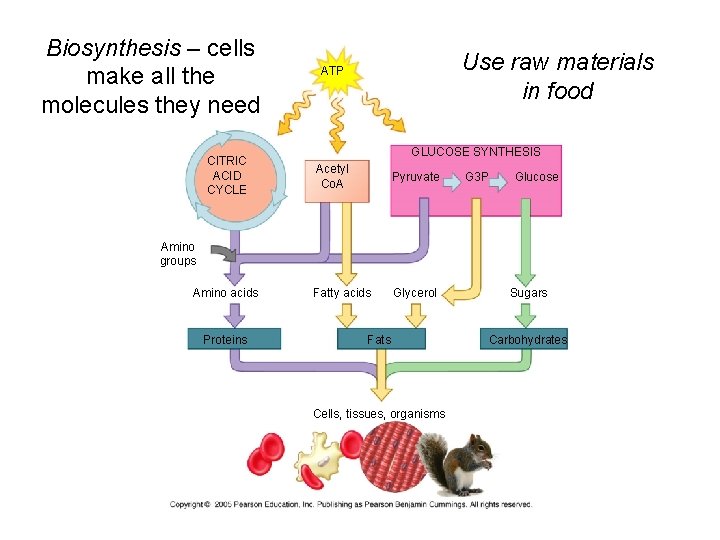
Biosynthesis – cells make all the molecules they need CITRIC ACID CYCLE Use raw materials in food ATP GLUCOSE SYNTHESIS Acetyl Co. A Pyruvate Fatty acids Glycerol G 3 P Glucose Amino groups Amino acids Proteins Fats Cells, tissues, organisms Sugars Carbohydrates

Cells make all the molecules they need using raw materials in food - biosynthesis 1. Not all food is used for energy 2. Cells can use monomers in food to make new molecules • Also use intermediate compounds in glycolysis and Kreb’s 3. can make molecules not found in food • Ex. Human protein from plant or animal protein 4. Biosynthesis uses ATP
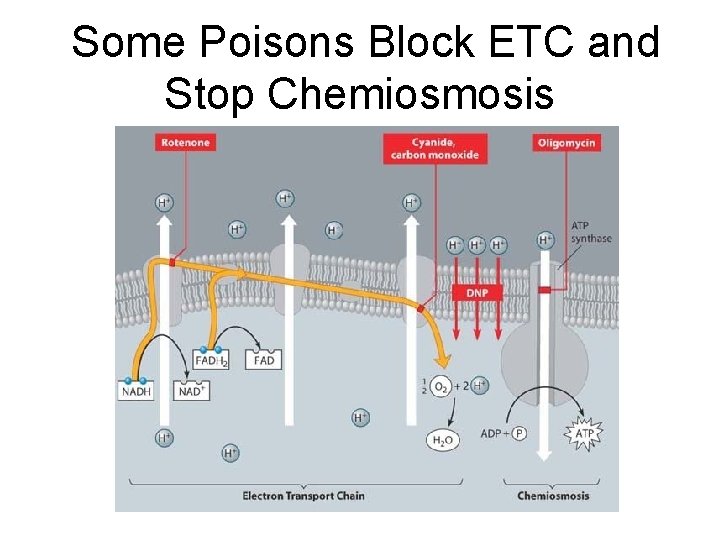
Some Poisons Block ETC and Stop Chemiosmosis
How Poisons Kill STOP H+ flow through ATP synthase a) Some block electron transfer b)Some don’t concentrate H+ no H+ gradient, no ATP
- Slides: 49