Homework 6 The compound HOCl hypochlorous acid reacts

- Slides: 13

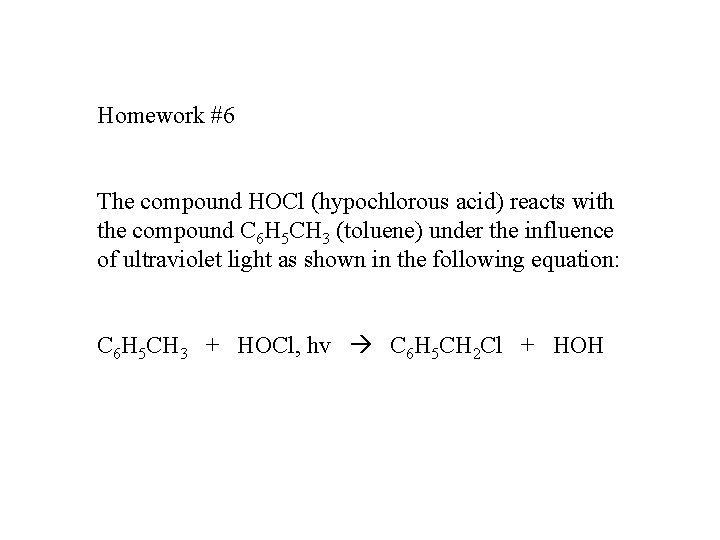
Homework #6 The compound HOCl (hypochlorous acid) reacts with the compound C 6 H 5 CH 3 (toluene) under the influence of ultraviolet light as shown in the following equation: C 6 H 5 CH 3 + HOCl, hv C 6 H 5 CH 2 Cl + HOH

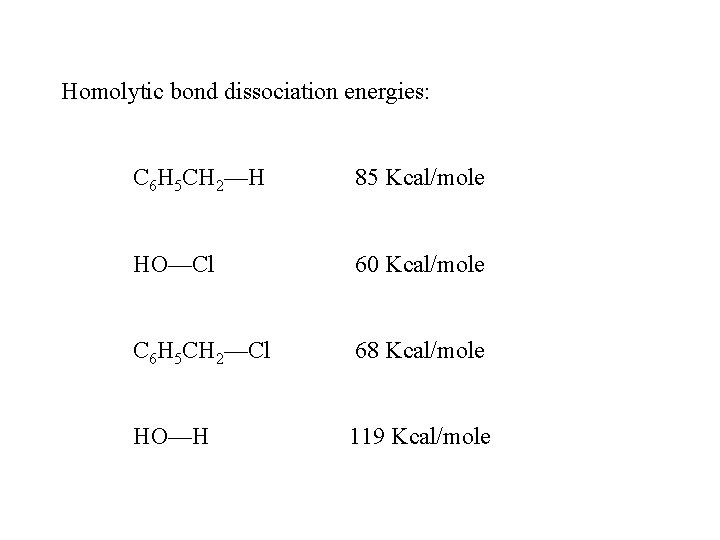
Homolytic bond dissociation energies: C 6 H 5 CH 2—H 85 Kcal/mole HO—Cl 60 Kcal/mole C 6 H 5 CH 2—Cl 68 Kcal/mole HO—H 119 Kcal/mole

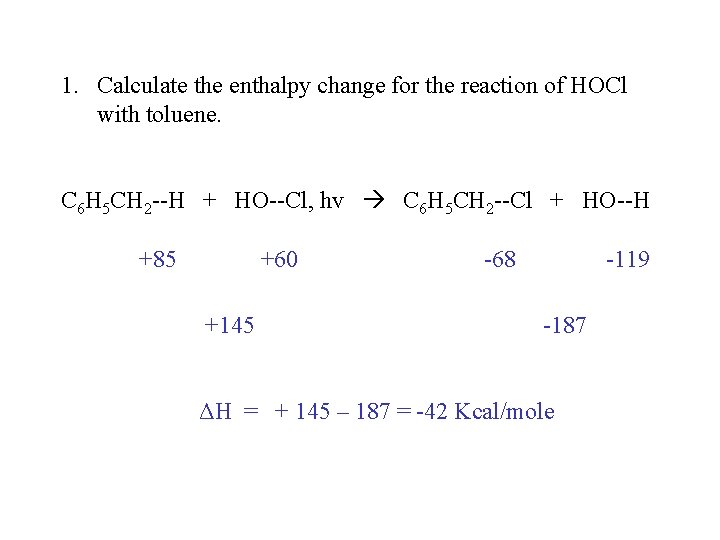
1. Calculate the enthalpy change for the reaction of HOCl with toluene. C 6 H 5 CH 2 --H + HO--Cl, hv C 6 H 5 CH 2 --Cl + HO--H +85 +60 +145 -68 -119 -187 ΔH = + 145 – 187 = -42 Kcal/mole

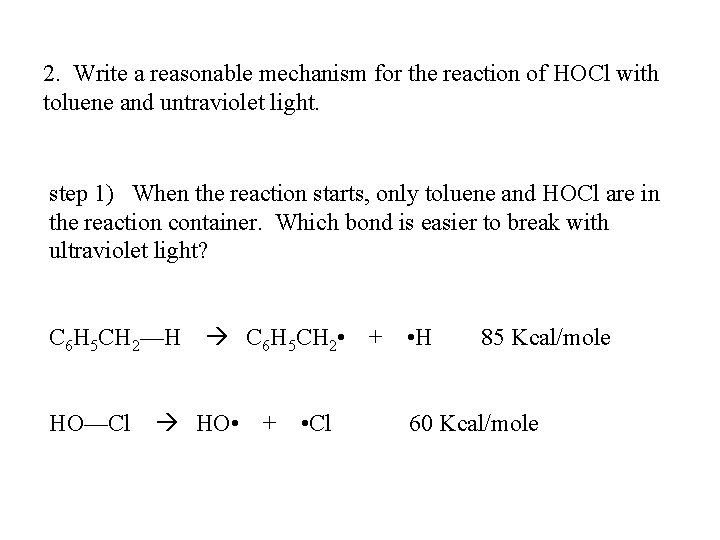
2. Write a reasonable mechanism for the reaction of HOCl with toluene and untraviolet light. step 1) When the reaction starts, only toluene and HOCl are in the reaction container. Which bond is easier to break with ultraviolet light? C 6 H 5 CH 2—H HO—Cl C 6 H 5 CH 2 • HO • + • Cl + • H 85 Kcal/mole 60 Kcal/mole

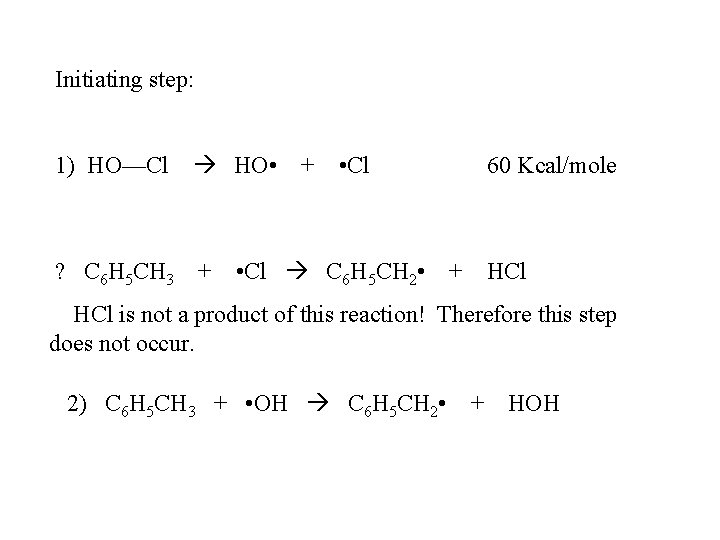
Initiating step: 1) HO—Cl HO • ? C 6 H 5 CH 3 + + • Cl C 6 H 5 CH 2 • 60 Kcal/mole + HCl is not a product of this reaction! Therefore this step does not occur. 2) C 6 H 5 CH 3 + • OH C 6 H 5 CH 2 • + HOH

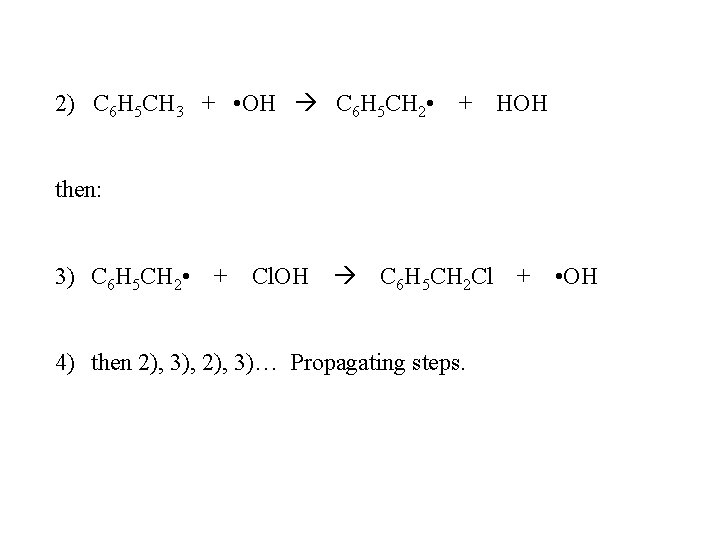
2) C 6 H 5 CH 3 + • OH C 6 H 5 CH 2 • + HOH then: 3) C 6 H 5 CH 2 • + Cl. OH C 6 H 5 CH 2 Cl + • OH 4) then 2), 3), 2), 3)… Propagating steps.

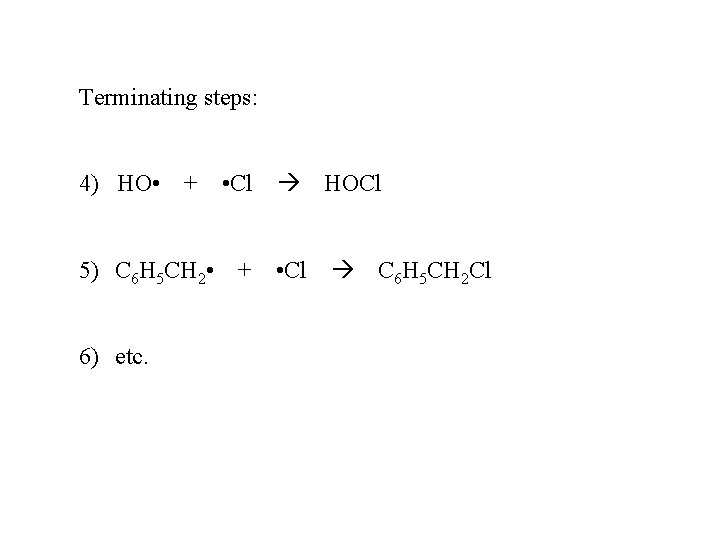
Terminating steps: 4) HO • + • Cl HOCl 5) C 6 H 5 CH 2 • + • Cl C 6 H 5 CH 2 Cl 6) etc.

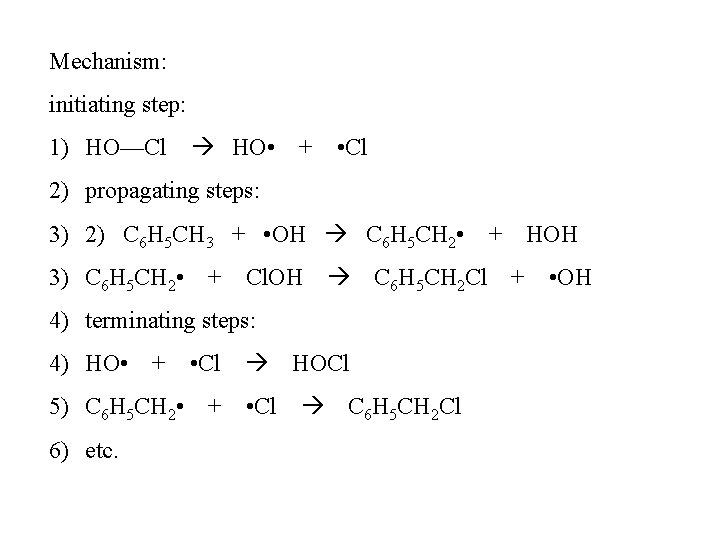
Mechanism: initiating step: 1) HO—Cl HO • + • Cl 2) propagating steps: 3) 2) C 6 H 5 CH 3 + • OH C 6 H 5 CH 2 • + HOH 3) C 6 H 5 CH 2 • + Cl. OH C 6 H 5 CH 2 Cl + • OH 4) terminating steps: 4) HO • + • Cl HOCl 5) C 6 H 5 CH 2 • + • Cl C 6 H 5 CH 2 Cl 6) etc.

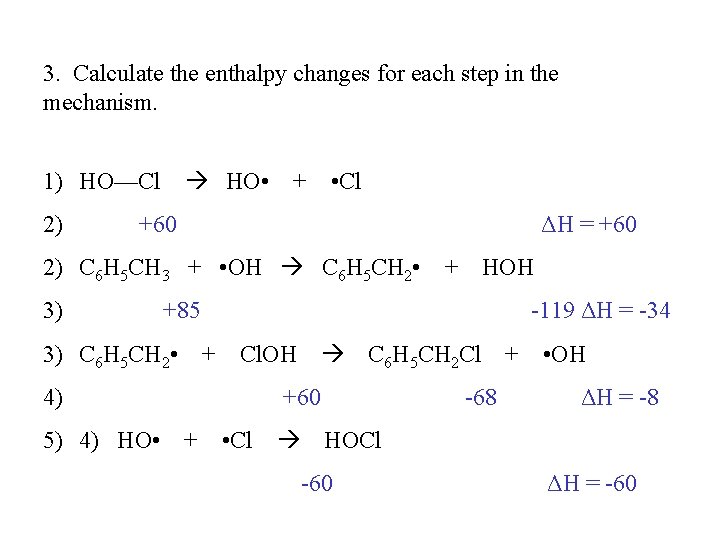
3. Calculate the enthalpy changes for each step in the mechanism. 1) HO—Cl HO • + • Cl 2) +60 ΔH = +60 2) C 6 H 5 CH 3 + • OH C 6 H 5 CH 2 • + HOH 3) +85 -119 ΔH = -34 3) C 6 H 5 CH 2 • + Cl. OH C 6 H 5 CH 2 Cl + • OH 4) +60 -68 ΔH = -8 5) 4) HO • + • Cl HOCl -60 ΔH = -60

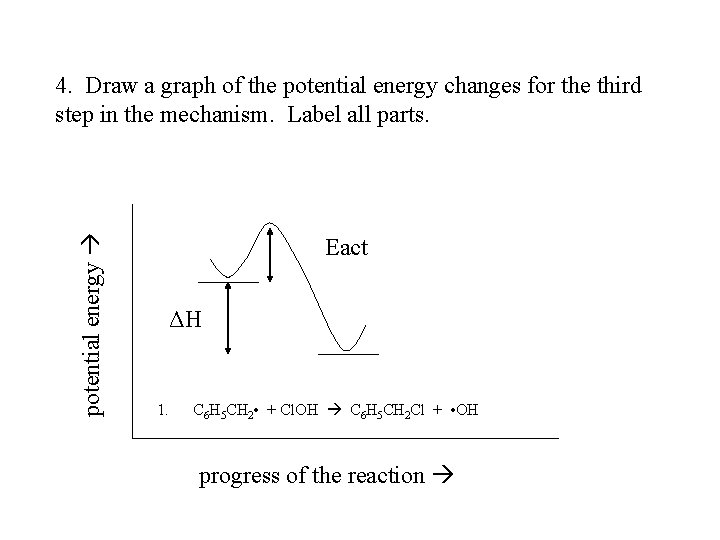
potential energy 4. Draw a graph of the potential energy changes for the third step in the mechanism. Label all parts. Eact ΔH 1. C 6 H 5 CH 2 • + Cl. OH C 6 H 5 CH 2 Cl + • OH progress of the reaction

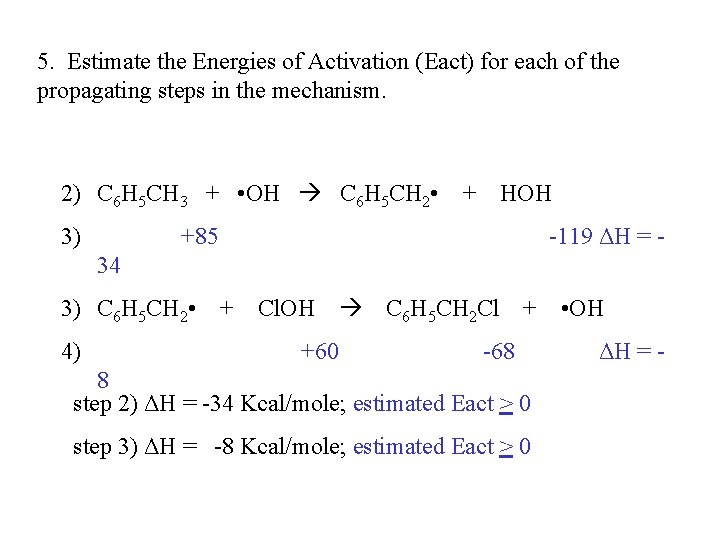
5. Estimate the Energies of Activation (Eact) for each of the propagating steps in the mechanism. 2) C 6 H 5 CH 3 + • OH C 6 H 5 CH 2 • + HOH 3) +85 -119 ΔH = - 34 3) C 6 H 5 CH 2 • + Cl. OH C 6 H 5 CH 2 Cl + • OH 4) +60 -68 8 step 2) ΔH = -34 Kcal/mole; estimated Eact > 0 step 3) ΔH = -8 Kcal/mole; estimated Eact > 0 ΔH = -

6. Which step in the mechanism is the rate determining step? In a chain mechanism, the rate determining step is the slowest propagating step in the mechanism. Which of the propagating steps is slower? step 2) ΔH = -34 Kcal/mole; estimated Eact > 0 step 3) ΔH = -8 Kcal/mole; estimated Eact > 0 Cannot tell from the available information!

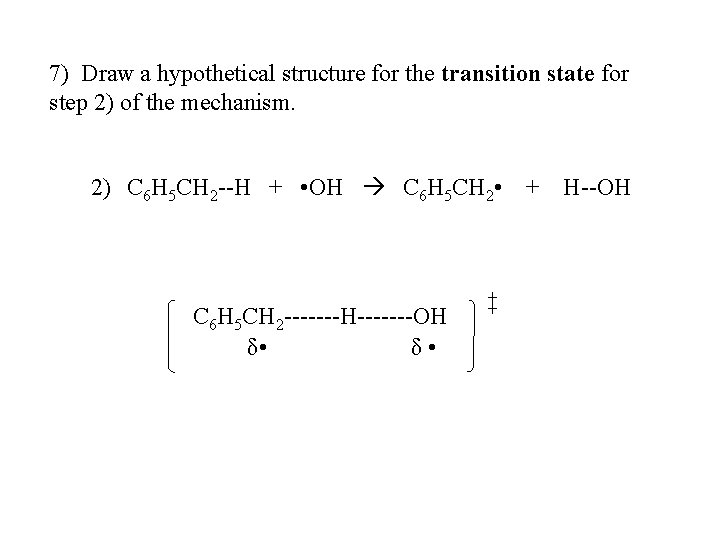
7) Draw a hypothetical structure for the transition state for step 2) of the mechanism. 2) C 6 H 5 CH 2 --H + • OH C 6 H 5 CH 2 • + H--OH C 6 H 5 CH 2 -------H-------OH δ • ‡