Homework 4 Charles Law WS Aim 4 How



























- Slides: 40

Homework # 4 • Charles’ Law WS

Aim 4: How is the volume of a gas affected when the temperature of the gas is changed?

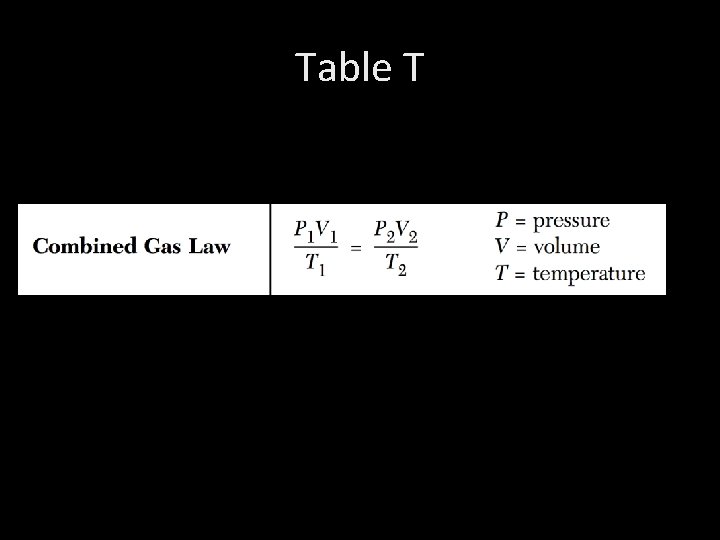
Table T

Charles’s Law • Discovered by French physicist, Jacques Charles in 1787

The relationship between temperature and volume

Do gases get heavier or lighter as their temperature rise?

Jacques Charles (1746 -1823) In the century following Boyle, a French physicist, Jacques Charles was the first person to fill a balloon with hydrogen gas and who made the first solo balloon flight.

If the temperature of a gas is increased, will its volume increase or decrease? Why?

Increasing the temperature: • Increases the kinetic energy • Increases collision (molecules collide more frequently!) • Increase the rate of reaction!

TEMPERATURE BALLOON IN A CUP OF HOT WATER BALLOON IN A CUP OF COLD WATER

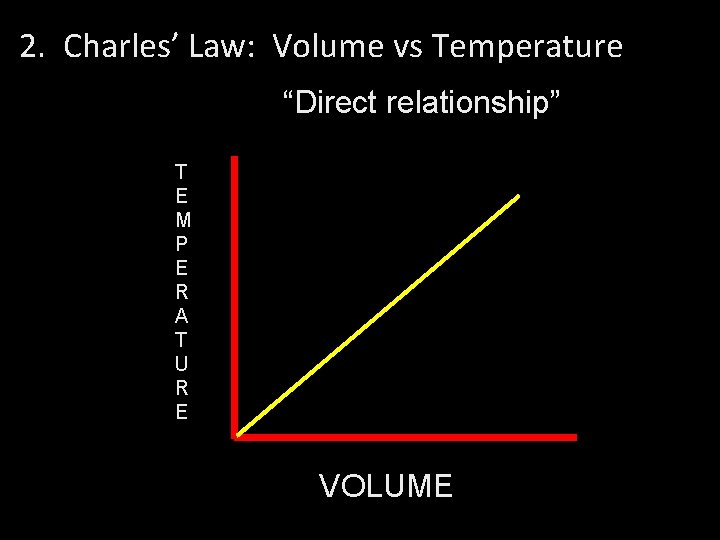
So, when the temperature of the gas increases its volume also increases. • What type of relationship exist between temperature and volume?

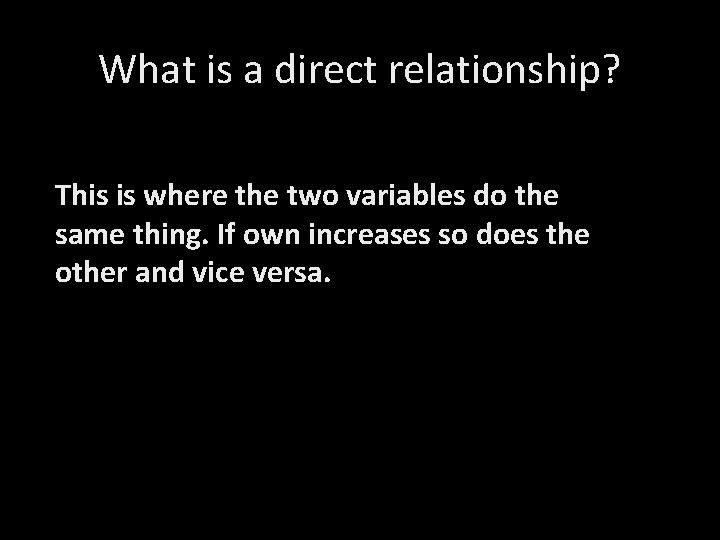
What is a direct relationship? This is where the two variables do the same thing. If own increases so does the other and vice versa.

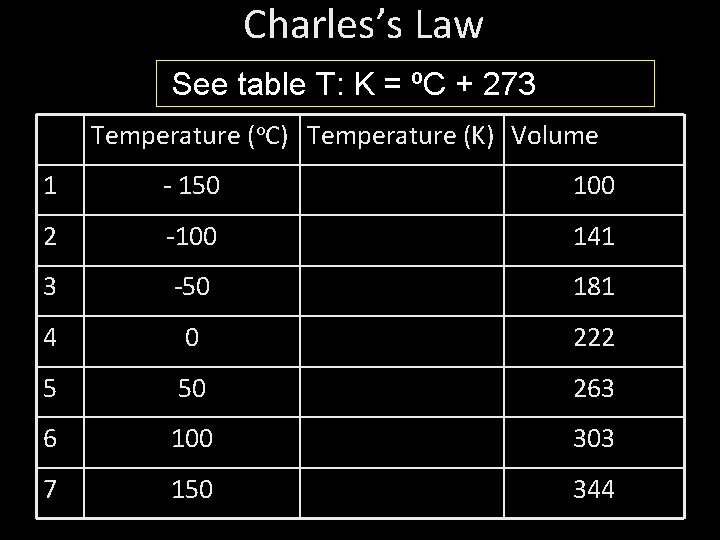
You need a calculator: How do you change -150 o. C to kelvin? See table T: K = ºC + 273

Charles’s Law See table T: K = ºC + 273 Temperature (o. C) Temperature (K) Volume 1 - 150 100 2 -100 141 3 -50 181 4 0 222 5 50 263 6 100 303 7 150 344

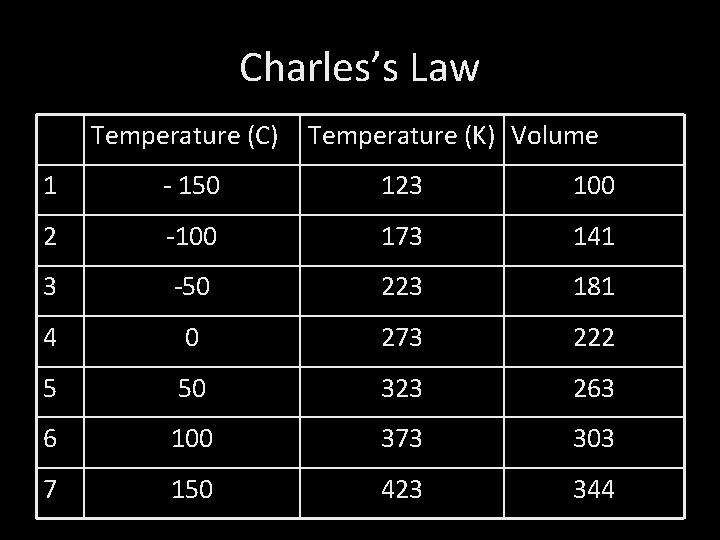
Charles’s Law Temperature (C) Temperature (K) Volume 1 - 150 123 100 2 -100 173 141 3 -50 223 181 4 0 273 222 5 50 323 263 6 100 373 303 7 150 423 344

Charles’s Law Temperature (K) Volume 1 123 100 2 173 141 3 223 181 4 273 222 5 323 263 6 373 303 7 423 344

1. Charles’s Law states that at constant pressure, volume is directly proportional to Kelvin temperature.
In math, how do you graph a direct relationship?


2. Charles’ Law: Volume vs Temperature “Direct relationship” T E M P E R A T U R E VOLUME

3. Combined Gas Law: See table T

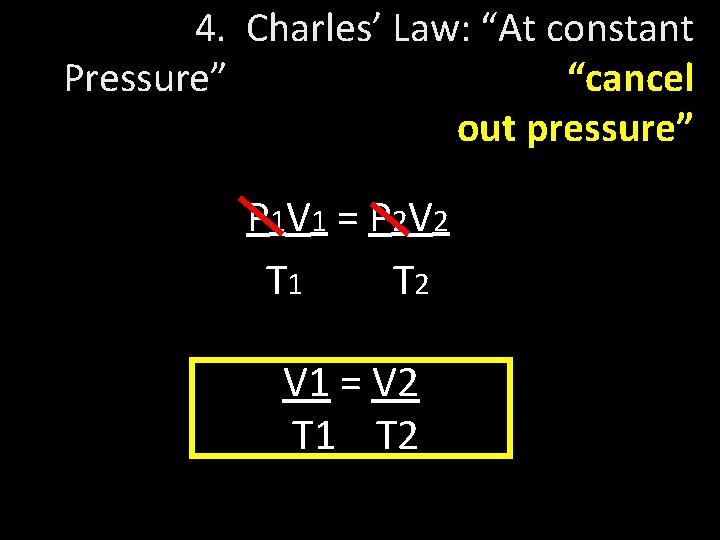
4. Charles’ Law: “At constant Pressure” “cancel out pressure” P 1 V 1 = P 2 V 2 T 1 T 2 V 1 = V 2 T 1 T 2

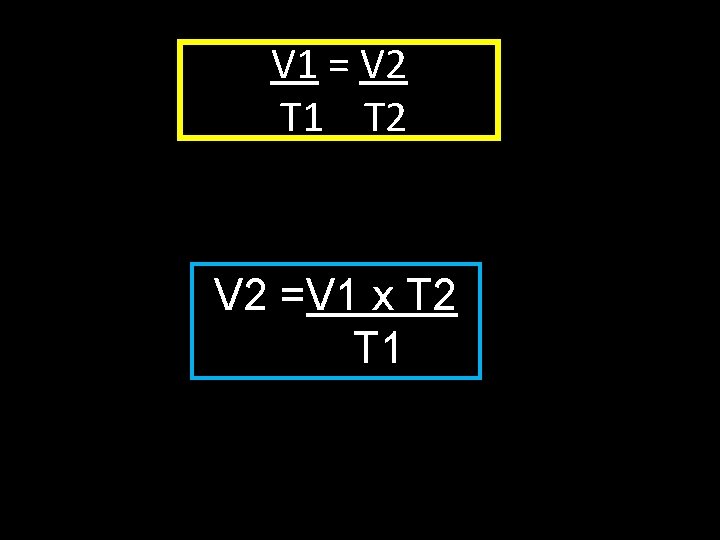
V 1 = V 2 T 1 T 2 Solve V 2 =V 1 x T 2 T 1

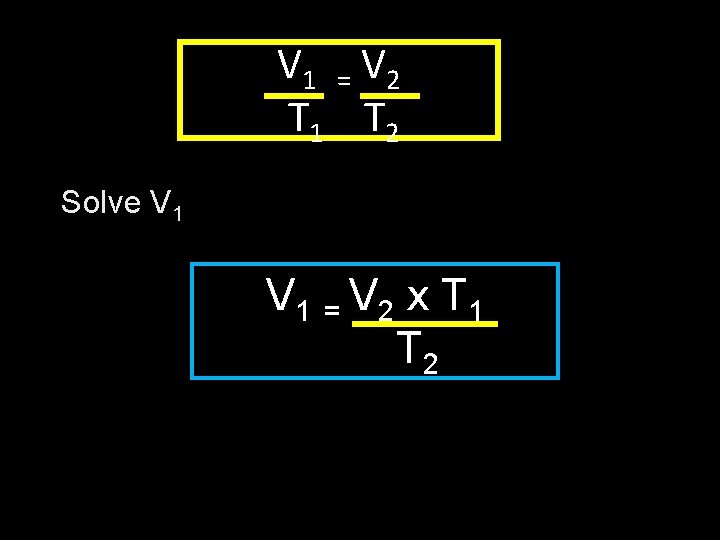
V 1 = V 2 T 1 T 2 Solve V 1 = V 2 x T 1 T 2

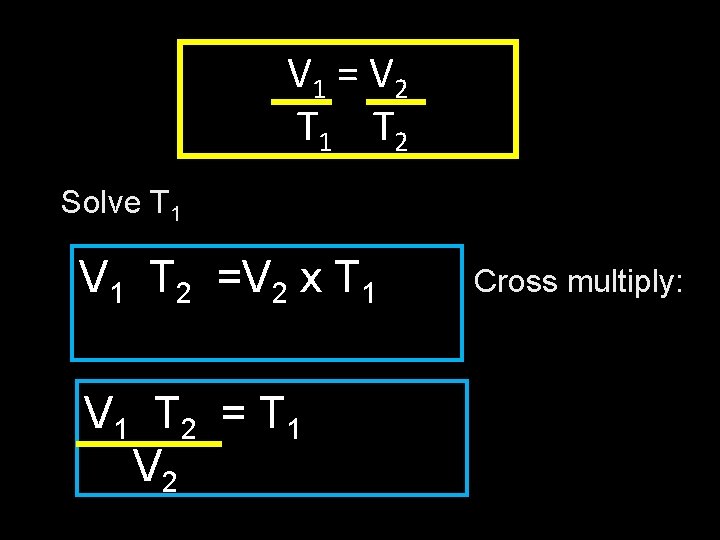
V 1 = V 2 T 1 T 2 Solve T 1 V 1 T 2 =V 2 x T 1 V 1 T 2 = T 1 V 2 Cross multiply:

Test Your Understanding

1. At 200 K, the volume of a gas is 100 m. L. The temperature is raised to 300 K. What is the new volume? Step 1: Data V 1= 100 m. L. T 1= 200 K V 2= X T 2= 300 K

V 1 = V 2 T 1 T 2 Step 2: Set up the problem: identify the data 100 m. L = V 2 200 K 300 K

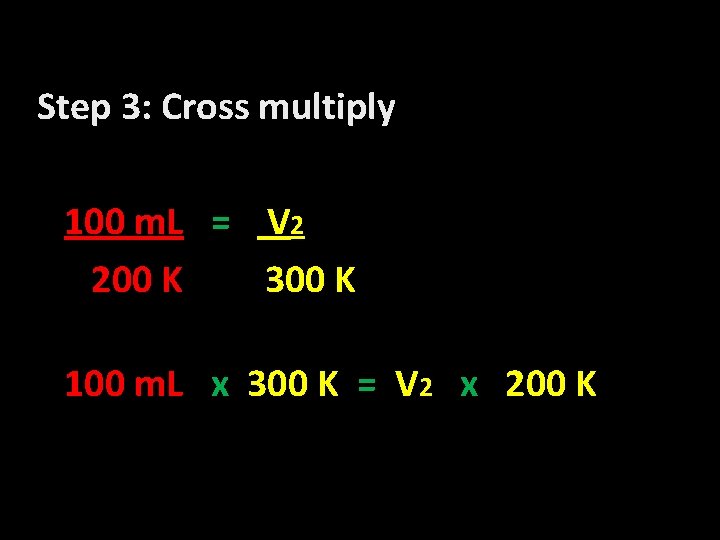
Step 3: Cross multiply 100 m. L = V 2 200 K 300 K 100 m. L x 300 K = V 2 x 200 K

Step 4: Solve V 2 100 m. L x 300 K = V 2 x 200 K 200 K 100 m. L x 300 = V 2 200 V 2= 150 m. L

V 1 = V 2 T 1 T 2 V 2 =V 1 x T 2 T 1

Solve V 2 100 m. L x 300 K = V 2 200 K 100 m. L x 300 = V 2 200 V 2= 150 m. L

2. A sample of a gas at 15°C has a volume of 2. 58 m. L. What volume will the gas occupy at 38°C ? Step 1: Data V 1= 2. 58 m. L V 2= X T 1 = 15 ºC T 2= 38 ºC

Remember: Temperature in Kelvin! Convert ºC to K ( See table T) K = ºC + 273 K = 15 + 273 = 288 K (Temperature 1) K = 38 + 273 = 311 K (Temperature 2)

Data: V 1= 2. 58 m. L V 2= X T 1 = 15 ºC = 288 K T 2= 38 ºC= 311 K

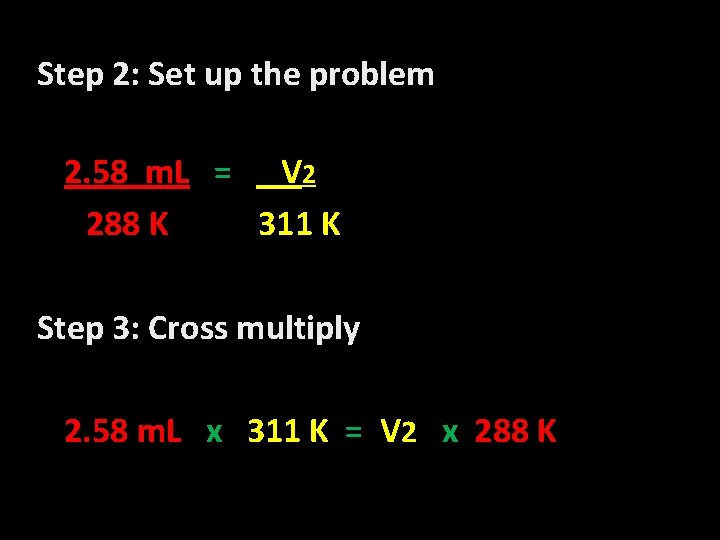
Step 2: Set up the problem 2. 58 m. L = V 2 288 K 311 K Step 3: Cross multiply 2. 58 m. L x 311 K = V 2 x 288 K

Step 4: Solve V 2 2. 58 m. L x 311 K = V 2 x 288 K 288 K Step 5: 2. 58 m. L x 311 = V 2 288 Step 6: V 2 = 2. 79 m. L

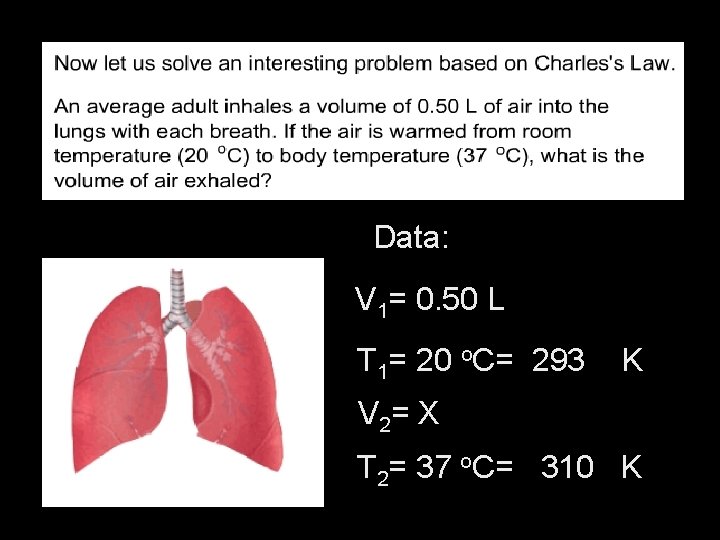
Data: V 1= 0. 50 L T 1= 20 o. C= 293 K V 2= X T 2= 37 o. C= 310 K

V 1 = V 2 T 1 T 2 0. 50 L = __X__ 293 K 310 K 0. 50 L x 310 K = 293 X 0. 50 L x 310 K = 293 K� X 293 K

END