Homework 3 due next Wednesday in class Homework




- Slides: 13

Homework 3 due next Wednesday in class Homework 4 posted- due Wednesday 14 Feb

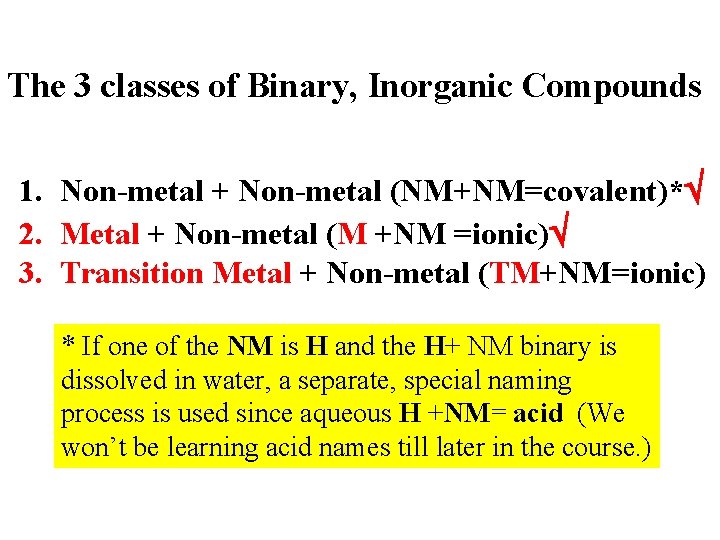
The 3 classes of Binary, Inorganic Compounds 1. Non-metal + Non-metal (NM+NM=covalent)* 2. Metal + Non-metal (M +NM =ionic) 3. Transition Metal + Non-metal (TM+NM=ionic) * If one of the NM is H and the H+ NM binary is dissolved in water, a separate, special naming process is used since aqueous H +NM= acid (We won’t be learning acid names till later in the course. )

TM +NM…almost like M + NM except that you have to specify the TM charge in the name (since TM comes in many flavors of charge) +3 -1 Example: Fe. Cl 3 Iron (III) Chloride

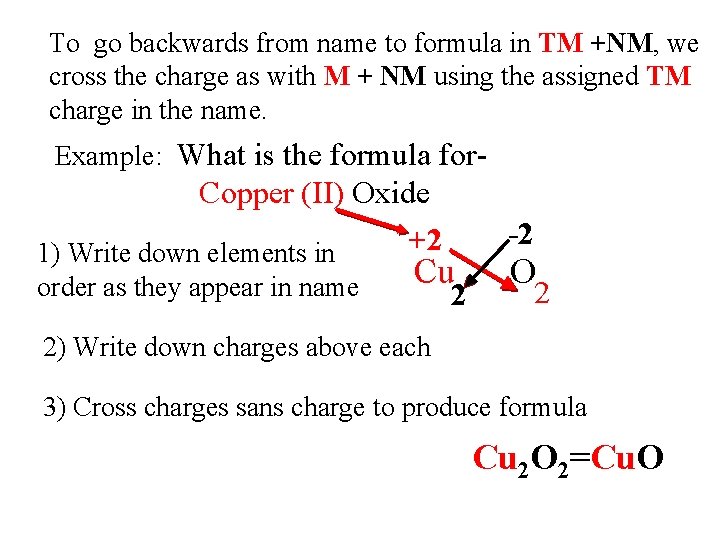
To go backwards from name to formula in TM +NM, we cross the charge as with M + NM using the assigned TM charge in the name. Example: What is the formula for- Copper (II) Oxide +2 1) Write down elements in order as they appear in name -2 Cu 2 O 2 2) Write down charges above each 3) Cross charges sans charge to produce formula Cu 2 O 2=Cu. O

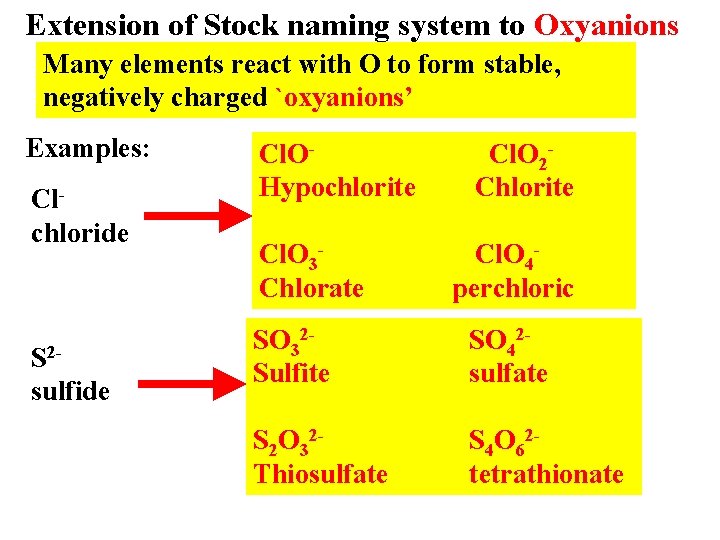
Extension of Stock naming system to Oxyanions Many elements react with O to form stable, negatively charged `oxyanions’ Examples: Clchloride S 2 sulfide Cl. OHypochlorite Cl. O 3 Chlorate Cl. O 2 Chlorite Cl. O 4 perchloric SO 32 Sulfite SO 42 sulfate S 2 O 32 Thiosulfate S 4 O 62 tetrathionate

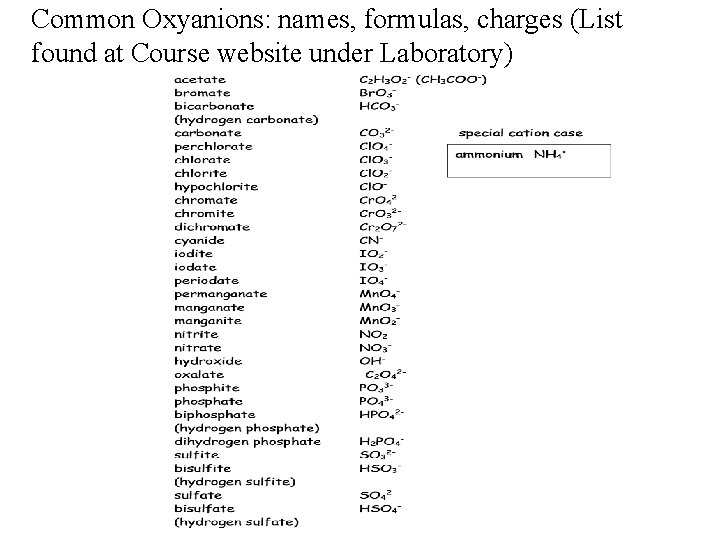
Common Oxyanions: names, formulas, charges (List found at Course website under Laboratory)

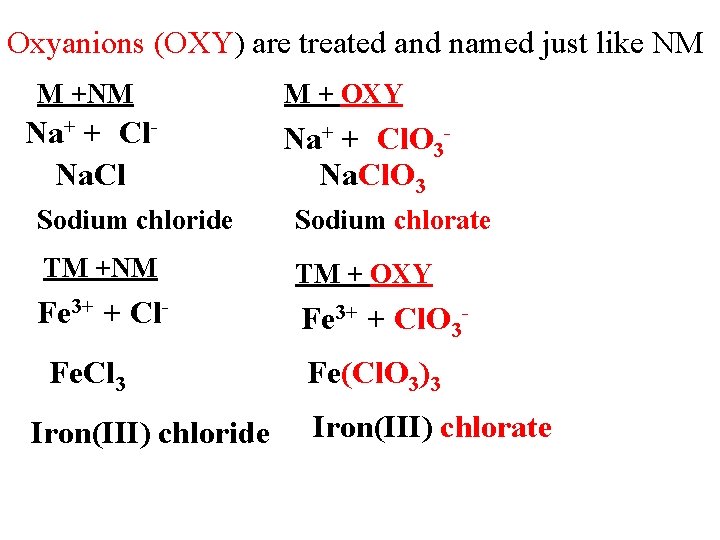
Oxyanions (OXY) are treated and named just like NM M +NM Na+ + Cl. Na. Cl M + OXY Na+ + Cl. O 3 Na. Cl. O 3 Sodium chloride Sodium chlorate TM +NM TM + OXY Fe 3+ + Cl- Fe 3+ + Cl. O 3 - Fe. Cl 3 Iron(III) chloride Fe(Cl. O 3)3 Iron(III) chlorate

In-class Board practice with OXYANIONS

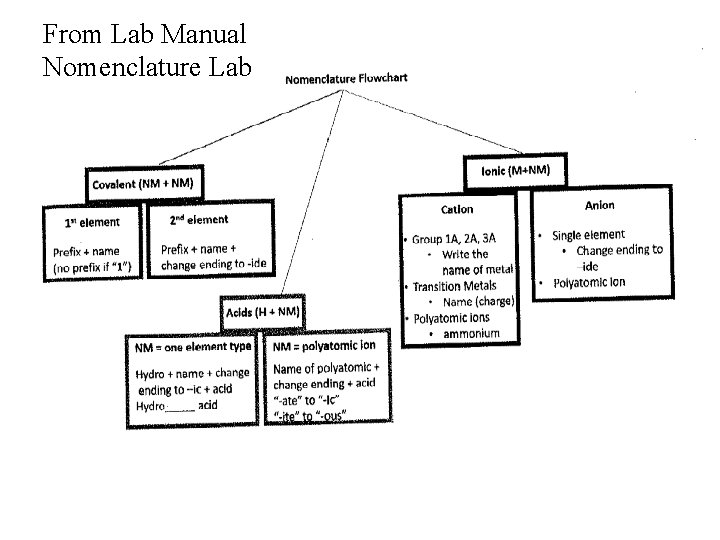
From Lab Manual Nomenclature Lab

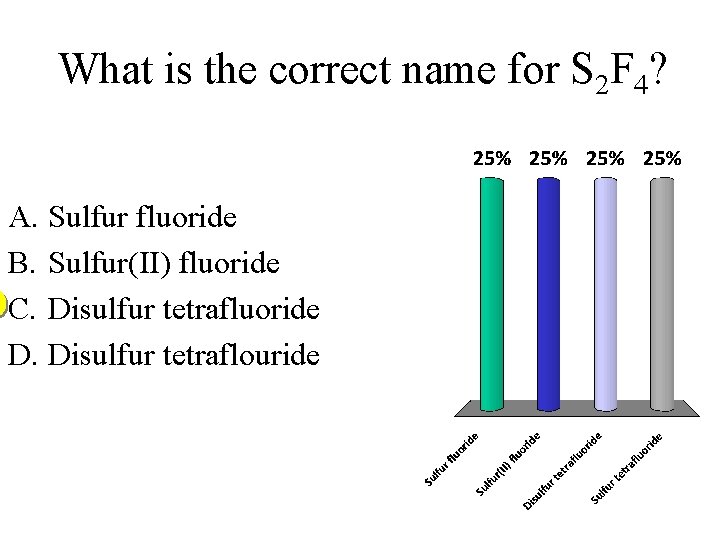
What is the correct name for S 2 F 4? A. Sulfur fluoride B. Sulfur(II) fluoride C. Disulfur tetrafluoride D. Disulfur tetraflouride

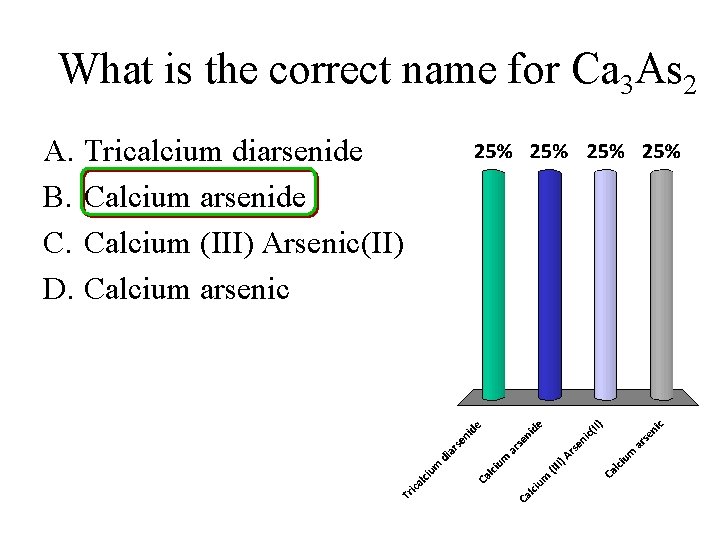
What is the correct name for Ca 3 As 2 A. Tricalcium diarsenide B. Calcium arsenide C. Calcium (III) Arsenic(II) D. Calcium arsenic

What is the correct name for Zn. O? A. Zinc(II) oxide B. Zinc monoxide C. Zinc oxide D. Monozinc monoxide

What is the correct formula for Copper(II) Acetate? A. Cu(C 2 H 3 O 2) B. Cu 2(C 2 H 3 O 2) C. Cu(C 2 H 3 O 2)2 D. Cu(C 3 H 2 O 3)