HN ADV CHEMISTRY CHAPTERS 1 4 Chapter 1















- Slides: 21

HN ADV CHEMISTRY CHAPTERS 1 - 4

Chapter 1: Chemical Foundations • 2 fundamental concepts of chemistry • 1. Matter is composed of various types of atoms • 2. One subs changes into another by reorganizing the way the atom are attached to each other • The SI System pg 8 & 9 • Table 1. 1 base units • Table 1. 2 prefixes • Uncertainty in Measurement • numbers obtained by using some measuring device • #’s that are the same regardless of who makes the measurement certain digits • last digit is estimated and varies w/the person who makes the measure uncertain digits

• all #’s have some degree of uncertainty amt depends on precision of device • significant figures/digits – all certain digits and the first uncertain digit • Pg 11 Sample exercise 1. 1 • Significant Figures and calculations • • pg. 13 rules for counting sig figs pg. 14 rules for sig figs in calculations and rounding • • • Precision & Accuracy accuracy – agreement of a value w/the true value precision – degree of agreement among several measurements of the same quantity – how close #’s are to each other Types of error 1. random error – (indeterminate error) – measurement has an equal probability of being high or low 2. systemic error – (determinate error) – occurs in same direction each time • • •

• Dimensional Analysis • used to convert one unit to another • uses conversion factors in ratio (want/have) – multiply by the #’s on top of ratio and divide by #’s on the bottom – make sure all units cancel until the last un-cancelled unit matches the unit of the answer • Density - ratio of a subs mass to its volume • D=m v

• • • Chapter 2: Atoms, Molecules and Ions Chemical Laws 1. Law of Conservation of Mass – mass is neither created nor destroyed it only changes form 2. Law of Definite Proportions – a given cmpd always contains exactly the same proportion of elements by mass proposed by Proust – Dalton proposed that matter is composed of atoms so a cmpd contains atoms in the same proportions 3. Law of Multiple Proportions – when 2 elements form a series of cmpds, the ratios of the masses of the 2 nd element that combine w/1 g of the 1 st can always be reduced to small whole #’s • Pg 42 Sample exercise 2. 1 • 4. Avogadro’s hypothesis – at the same temp & pressure, equal volumes of different gases contain the same # of particles • • Subatomic Particles Mass Charge Electron 9. 11 x 10 -31 kg -1 Proton 1. 67 x 10 -27 kg +1 Neutron 1. 67 x 10 -27 kg none One atom turns to another and says, “Darn it. I’ve lost an electron!” The other atom says, “Are you sure? ” and the first says, “I’m positive!”

• • • Molecules and Ions - chemical bonds – forces that hold atoms together - covalent bonds – e- shared – particle formed called a molecule – molecules represented by chemical formula or structural formula - ionic bonds – particles exchange e- to form charged particles ions (+) ion = cation (-) ion = anion Chapter 3: Stoichiometry • Atomic masses - based on 612 C as std – mass = 12 amu • - mass spectrometer is used to compare masses of atoms • - given on periodic table avg atomic mass of all naturally occurring isotopes • - can use a mass spec to determine the isotopic composition of a natural element • Pg 81 Sample exercise 3. 1

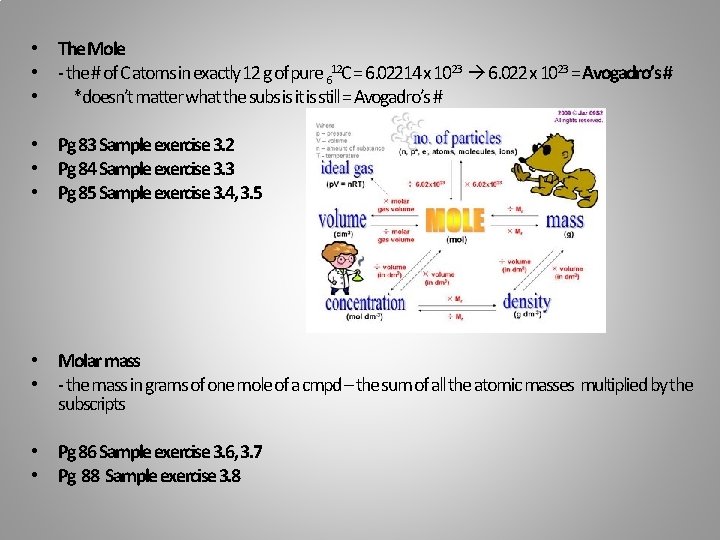
• The Mole • - the # of C atoms in exactly 12 g of pure 612 C = 6. 02214 x 1023 6. 022 x 1023 = Avogadro’s # • *doesn’t matter what the subs is it is still = Avogadro’s # • Pg 83 Sample exercise 3. 2 • Pg 84 Sample exercise 3. 3 • Pg 85 Sample exercise 3. 4, 3. 5 • Molar mass • - the mass in grams of one mole of a cmpd – the sum of all the atomic masses multiplied by the subscripts • Pg 86 Sample exercise 3. 6, 3. 7 • Pg 88 Sample exercise 3. 8

• • Percent composition - compare the mass of each element to the total mass of the cmpd mass percent • Pg 89 Sample exercise 3. 9 • • Determining the formula of a cmpd - need to determine the % mass of each element in the cmpd - empirical formula – smallest whole # ratio of elements in a cmpd - molecular formula – some multiple of the emp. formula based on the ratio Molecular molar mass = multiple of Empirical molar mass empirical • Pg 93 Sample exercise 3. 11 • • • Chemical equations - depict chem rxns - Reactants Products

• - bonds have been broken and reformed to produce new subs • - must show that all atoms in reactants are accounted for in products by balancing equation (law of conservation of matter) • - shows states of matter • - shows # of atoms/molecules in rxn • - shows # of moles of R and P in rxn • Pg 100 Sample exercise 3. 14 • • Stoichiometric Calculations: 1. Balanced equation 2. State given 3. State what is wanted 4. Convert given to moles 5. Mole to Mole ratio (from balanced equation) 6. Convert to units of answer

• Pg 105 Sample exercise 3. 16, 3. 17 • **any step not needed just SKIP • • Limiting Reactants - the reactant consumed firsts limits the amt of product formed - to determine the LR do a stoichiometry problem starting w/1 R and end w/the other - compare to amts given - to determine amt of product start w/LR and end w/product • Pg 110 Sample exercise 3. 18 • • Percent yield - amt of product formed when a reactant is completely consumed is called theoretical yield - amt of product formed in reality is called the actual yield - these can be used to determine the % yield • • • Actual x 100 = % yield Theoretical Pg 111 Sample exercise 3. 19

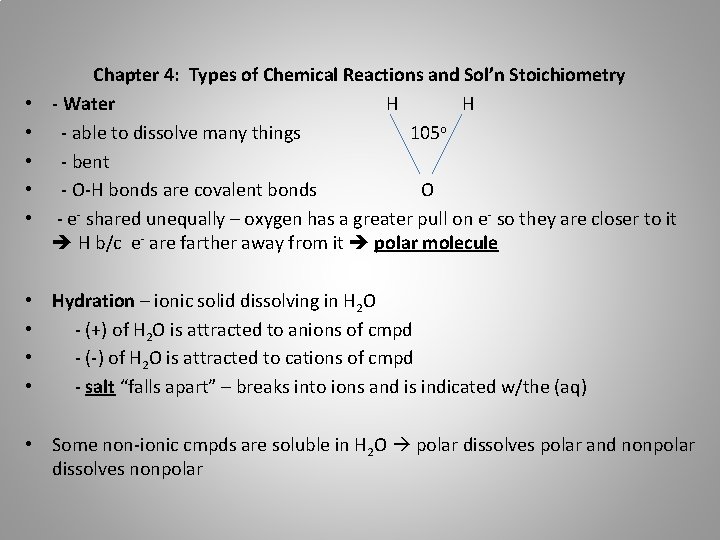
• • • Chapter 4: Types of Chemical Reactions and Sol’n Stoichiometry - Water H H - able to dissolve many things 105 o - bent - O-H bonds are covalent bonds O - e- shared unequally – oxygen has a greater pull on e- so they are closer to it H b/c e- are farther away from it polar molecule • Hydration – ionic solid dissolving in H 2 O • - (+) of H 2 O is attracted to anions of cmpd • - (-) of H 2 O is attracted to cations of cmpd • - salt “falls apart” – breaks into ions and is indicated w/the (aq) • Some non-ionic cmpds are soluble in H 2 O polar dissolves polar and nonpolar dissolves nonpolar

• • • • Sol’ns - made up or solutes and solvents - some can conduct electricity electrolytes - strong electrolytes – conduct efficiently - weak electrolytes – conduct poorly - non-electrolytes – do not conduct - conductivity is a factor of how many ions are present in a sol’n - strong electrolytes ionize completely - soluble salts are easily hydrated - strong acids produce H+ and other ions - all form (aq) sol’n - completely dissociate into ions - exception: H 2 SO 4 , only 1 H+ dissociates - strong bases release OH- from cations

• • • - weak electrolytes have only a small amt of ionization - weak acids contain an acidic H+ and nonacidic H’s – ex: H 3 PO 4 - weak bases partially lose OH- in sol’n • - nonelectrolytes – dissolve in H 2 O but do not produce any ions • • Composition of sol’ns - need to know 2 things to do Stoichiometric calcs 1. nature of reactants 2. amts of chem in sol’n • • - Molarity – (M) - expression of sol’n concentration • • M = moles of solute L of sol’n Pg 134 Sample exercise 4. 1, 4. 2, 4. 3 Pg 135 Sample exercise 4. 4, 4. 5

• Std sol’n – sol’n whose conc. Is accurately known • • • Pg 136 Sample exercise 4. 6 • • Dilutions - sol’ns are purchased in conc. form and H 2 O is added to desired molarity • Std sol’n – sol’n whose conc. Is accurately known M 1 V 1 = M 2 V 2 • - when solving for volume of conc. sol’n, remember that H 2 O must be added to reach the total volume needed • Pg 138 Sample exercise 4. 7

• • • Types of Chem Rxns - Precipitation Rxns – 2 sol’ns are mixed and a solid forms and separates from sol’n(precipitate, ppt) - products must have a net charge of 0 – (+) = (-) - rules for determining solubilities on pg. 144 Table 4. 1 - slightly soluble – a tiny amt of solid dissolves (not noticeable) sometimes insoluble • Pg 144 Sample exercise 4. 8 • - equations so far have been molecular equations – do not show what is happening b/n particles in the sol’n • • Ex: K 2 Cr. O 4(aq) + Ba(NO 3)2(aq) Ba. Cr. O 4(s) + 2 KNO 3(aq) - complete ionic equations – all subs that are strong electrolytes are represented as ions • Ex: 2 K+ (aq) + Cr. O 42 -(aq) + Ba 2+ (aq) + 2 NO 3 - (aq) Ba. Cr. O 4(s) + 2 K+ (aq) + 2 NO 3 - (aq) • *not all ions participate in the rxn spectator ions, e. g. K+ NO 3 -

• • - net ionic equations – includes only those ions that participate in rxn Ex: Ba 2+(aq) + Cr. O 4 2 -(aq) Ba. Cr. O 4(s) • Pg 146 Sample exercise 4. 9 • • • Stoichiometry of ppt rxns - must decide what will occur when 2 sol’ns are mixed – write down species (ions) in sol’n - to obtain moles of reactants use volume of sol’n and Molarity • • Pg 147 Sample exercise 4. 10 *Steps (1 -6) pg 148 • Pg 148 Sample exercise 4. 11 • • Acid-base rxns - according to Bronstead-Lowry - acid is a proton (H+) donor - base is a proton (H+) acceptor

• • *OH- is such a strong base that for stoichiometry calcs. It can be assumed to react completely w/any weak acid - rxn is called a neutralization rxn • Pg 150 Sample exercise 4. 12 • Pg 151 Sample exercise 4. 13 • Acid-base titrations • - volumetric analysis – technique for determining the amt of a certain subs by doing a titration – delivery (from a buret) of a measured volume of a sol’n a known conc. (the titrant) into a sol’n containing the sub being analyzed (the analyte) - when exactly enough titrant has been added to react w/the analyte called the equivalence point or stoichiometric point *marked by an indicator – a subs that changes color according to p. H • - point at which indicator changes color called the endpoint • *you need to choose an indicator that changes color at the equivalence point

• • • Requirements for successful titration: 1. Exact rxn b/n titrant and analyte must be known 2. Stoichiometric/equivalence point must be marked accurately 3. Volume of titrant required to reach Stoichiometric point must be known *Use strong acids/bases for titrant • Standardizing the sol’n • Pg 153 Sample exercise 4. 14, 4. 15 • • • Redox rxns - one or more e- are transferred b/n subs - most energy producing rxns are redox rxns - oxidation states/oxidation #’s – help track e- in redox rxns *Rules for assigning oxidation #’s pg 156 Table 4. 2 • Pg 157 Sample exercise 4. 16

• • **Exception: +2/3 -2 Fe O 4 +8 -8 - b/c it’s an “avg” - some Fe’s are 2+ and some are 3+ *occurs in other cmpds DON’T FREAK!! • • -4+1 0 +4 -2 +1 -2 Ex: CH 4 + 2 O 2 CO 2 + 2 H 2 O Oxidation loss 8 e- RA Reduction gained 2 e- OA • *if a subs is reduced it ---------------------- *if a subs is oxidized it causes oxidation in the -7 -6 -5 -4 -3 -2 -10+1+2+3+4+5+6+7 causes reduction in the other oxidizing agent other reducing agent gain of eloss of ereduction oxidation • • pg 160 Sample exercise 4. 17 Pg 161 Sample exercise 4. 18

• • Balancing Redox Rxns - use half-rxn method - separate into one for oxidation and one for reduction Ex: Ce 4+ + Sn 2+ Ce 3+ + Sn 4+ Reduction Ce 4+ Ce 3+ 2[1 e- + Ce 4+ Ce 3+] 2 e- + 2 Ce 4+ 2 Ce 3+ Oxidation Sn 2+ Sn 4+ + 2 e- • • • 2 Ce 4+ + Sn 2+ 2 Ce 3+ + Sn 4+ 2(4+) + 2 2(3+) + 4 +10 • *pg 162 Steps for balancing Redox rxns using half rxns

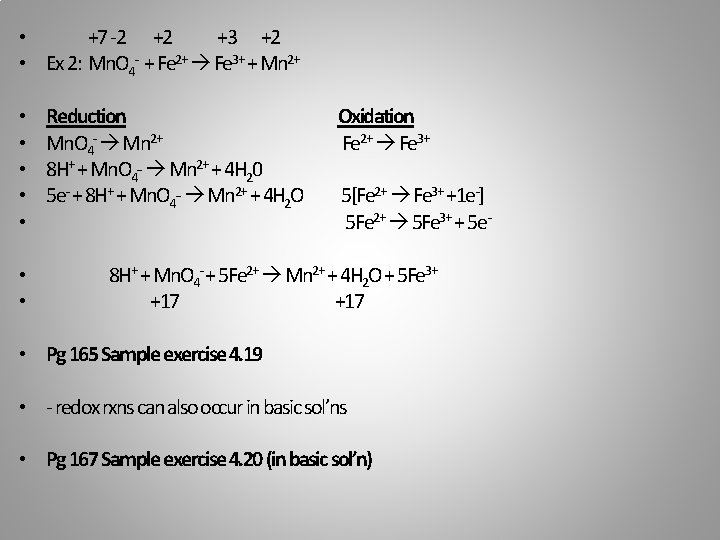
• +7 -2 +2 +3 +2 • Ex 2: Mn. O 4 - + Fe 2+ Fe 3+ + Mn 2+ • • Reduction Mn. O 4 - Mn 2+ 8 H+ + Mn. O 4 - Mn 2+ + 4 H 20 5 e- + 8 H+ + Mn. O 4 - Mn 2+ + 4 H 2 O Oxidation Fe 2+ Fe 3+ 5[Fe 2+ Fe 3+ +1 e-] 5 Fe 2+ 5 Fe 3+ + 5 e- 8 H+ + Mn. O 4 - + 5 Fe 2+ Mn 2+ + 4 H 2 O + 5 Fe 3+ +17 • Pg 165 Sample exercise 4. 19 • - redox rxns can also occur in basic sol’ns • Pg 167 Sample exercise 4. 20 (in basic sol’n)