HL Organic Chemistry NUCLEOPHILIC SUBSTITUTION REACTIONS Types of
HL Organic Chemistry: NUCLEOPHILIC SUBSTITUTION REACTIONS
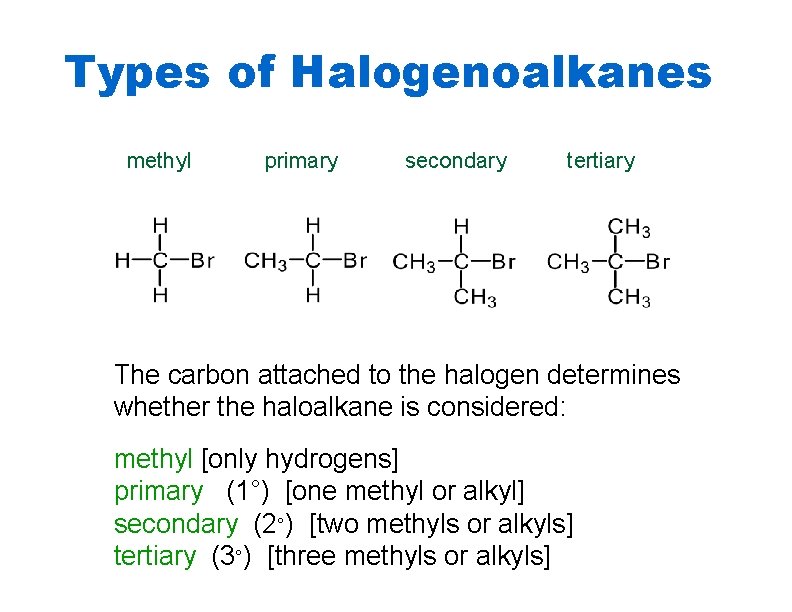
Types of Halogenoalkanes methyl primary secondary tertiary The carbon attached to the halogen determines whether the haloalkane is considered: methyl [only hydrogens] primary (1°) [one methyl or alkyl] secondary (2°) [two methyls or alkyls] tertiary (3°) [three methyls or alkyls]
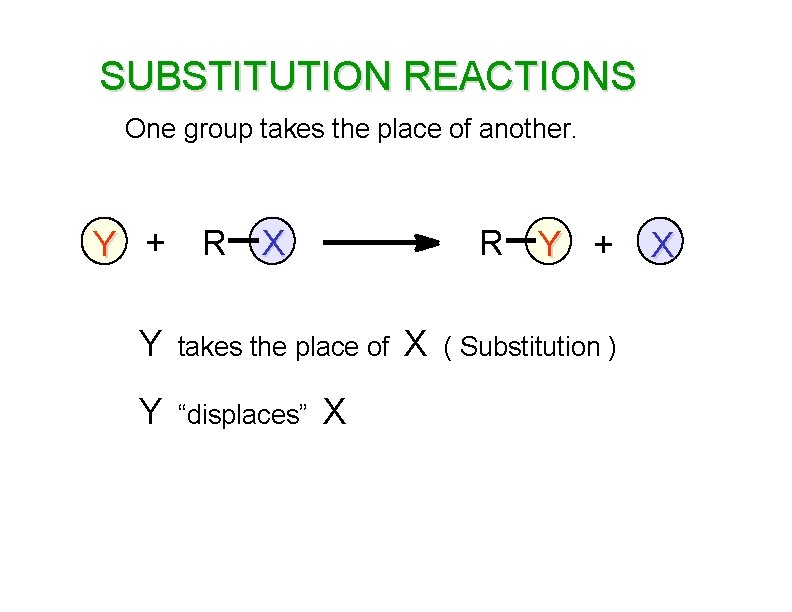
SUBSTITUTION REACTIONS One group takes the place of another. Y + R X R Y + Y takes the place of Y “displaces” X X ( Substitution ) X
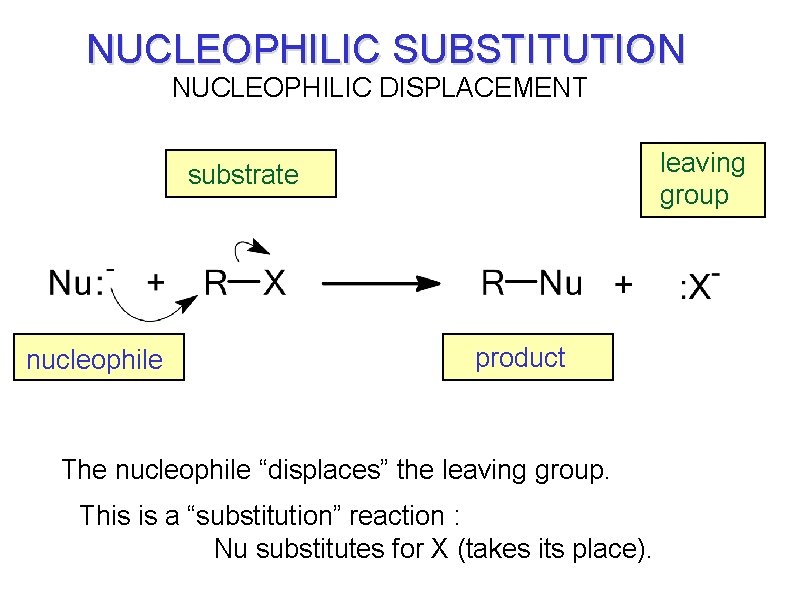
NUCLEOPHILIC SUBSTITUTION NUCLEOPHILIC DISPLACEMENT leaving group substrate nucleophile product The nucleophile “displaces” the leaving group. This is a “substitution” reaction : Nu substitutes for X (takes its place).
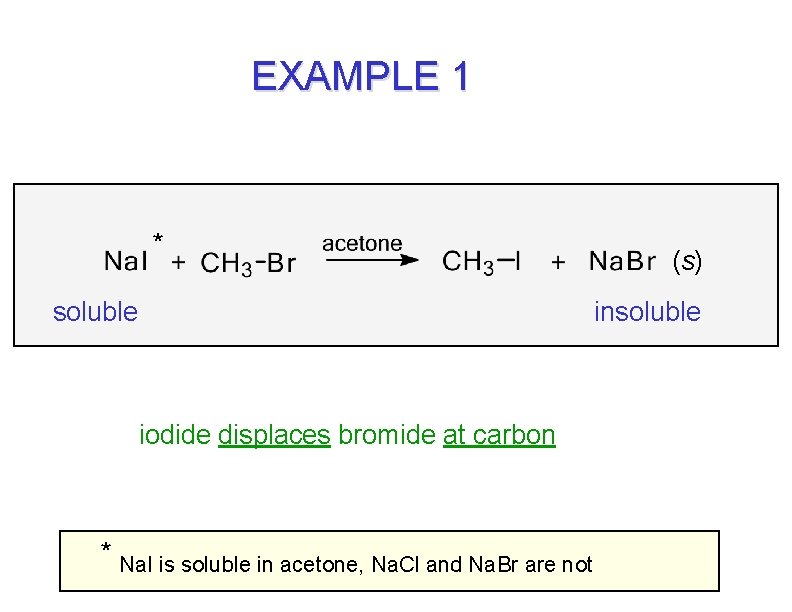
EXAMPLE 1 * soluble (s) insoluble iodide displaces bromide at carbon * Na. I is soluble in acetone, Na. Cl and Na. Br are not
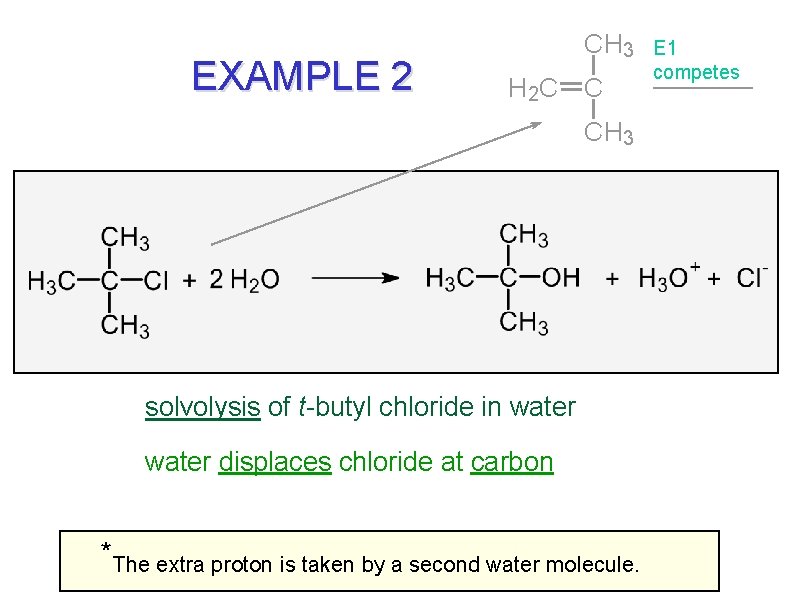
EXAMPLE 2 CH 3 H 2 C C CH 3 solvolysis of t-butyl chloride in water displaces chloride at carbon * The extra proton is taken by a second water molecule. E 1 competes
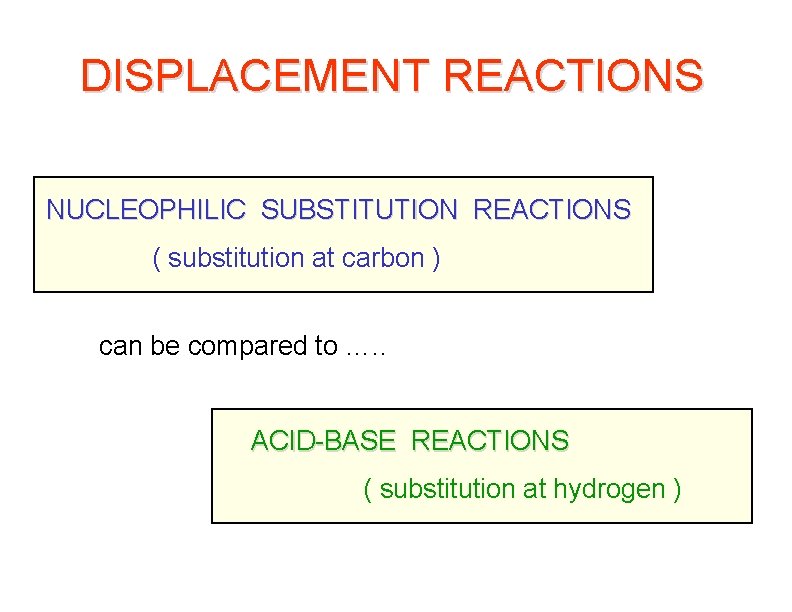
DISPLACEMENT REACTIONS NUCLEOPHILIC SUBSTITUTION REACTIONS ( substitution at carbon ) can be compared to …. . ACID-BASE REACTIONS ( substitution at hydrogen )
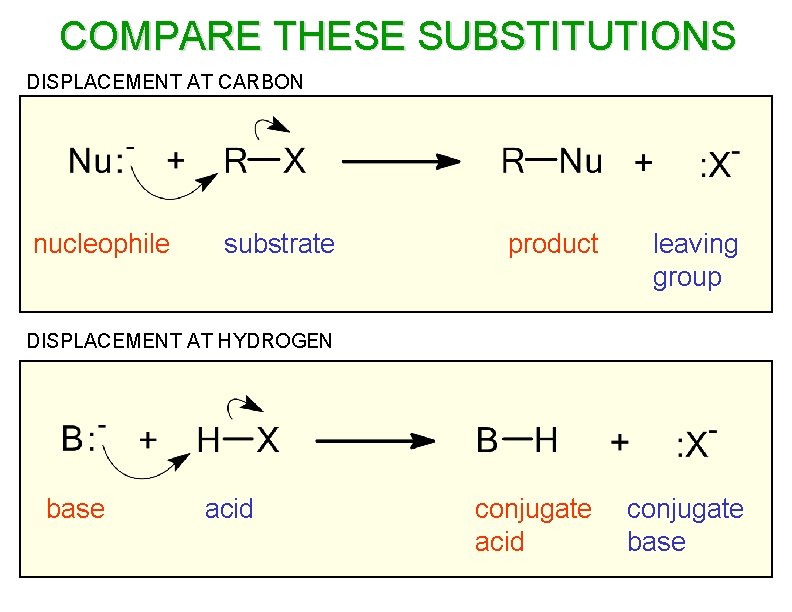
COMPARE THESE SUBSTITUTIONS DISPLACEMENT AT CARBON nucleophile substrate product leaving group conjugate acid conjugate base DISPLACEMENT AT HYDROGEN base acid

THESE REACTIONS HAVE A WIDE RANGE OF SUBSTRATES
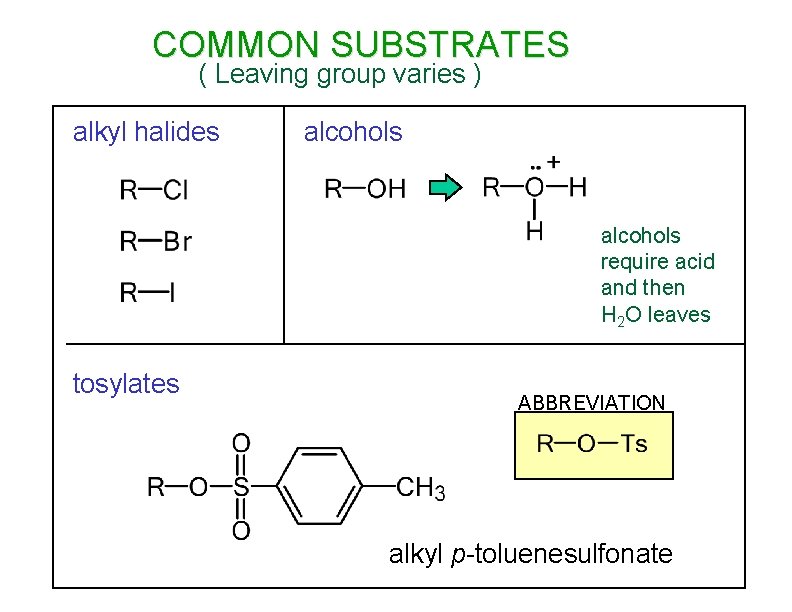
COMMON SUBSTRATES ( Leaving group varies ) alkyl halides alcohols require acid and then H 2 O leaves tosylates ABBREVIATION alkyl p-toluenesulfonate

THERE ALSO A WIDE RANGE OF NUCLEOPHILES A WIDE VARIETY OF COMPOUNDS CAN BE MADE
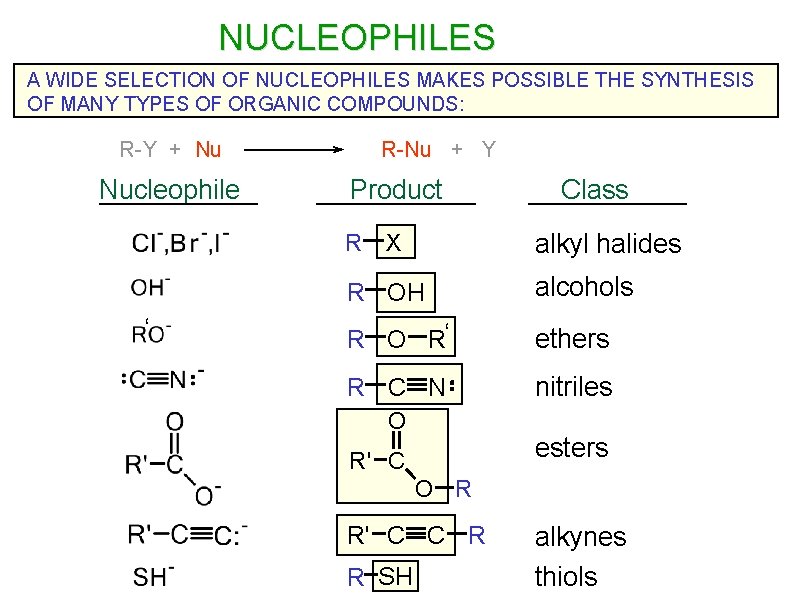
NUCLEOPHILES A WIDE SELECTION OF NUCLEOPHILES MAKES POSSIBLE THE SYNTHESIS OF MANY TYPES OF ORGANIC COMPOUNDS: R-Y + Nu Nucleophile R-Nu + Y Product R ‘ Class alkyl halides X R OH alcohols R O R‘ ethers R C N nitriles O esters R' C O R R' C C R alkynes R SH thiols
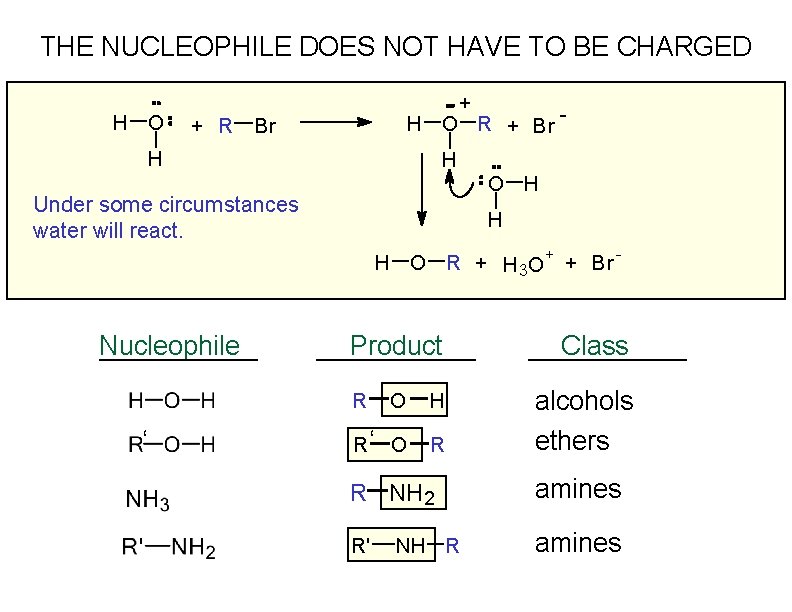
THE NUCLEOPHILE DOES NOT HAVE TO BE CHARGED H O + H O R + Br - + R Br H H O H Under some circumstances water will react. H H O R + H 3 O + + Br Nucleophile Product Class O H alcohols R‘ O R ethers R ‘ - R NH 2 amines R' amines NH R

REACTION 1 THE SN 2 REACTION
![55 o. C rate = k 2 [RBr] [Na. OH] k 2 = 0. 55 o. C rate = k 2 [RBr] [Na. OH] k 2 = 0.](http://slidetodoc.com/presentation_image_h2/72239d303572349bdb616b33283bfdfb/image-15.jpg)
55 o. C rate = k 2 [RBr] [Na. OH] k 2 = 0. 022 liter/mole-sec - bimolecular concerted S N 2 substitution nucleophilic bimolecular
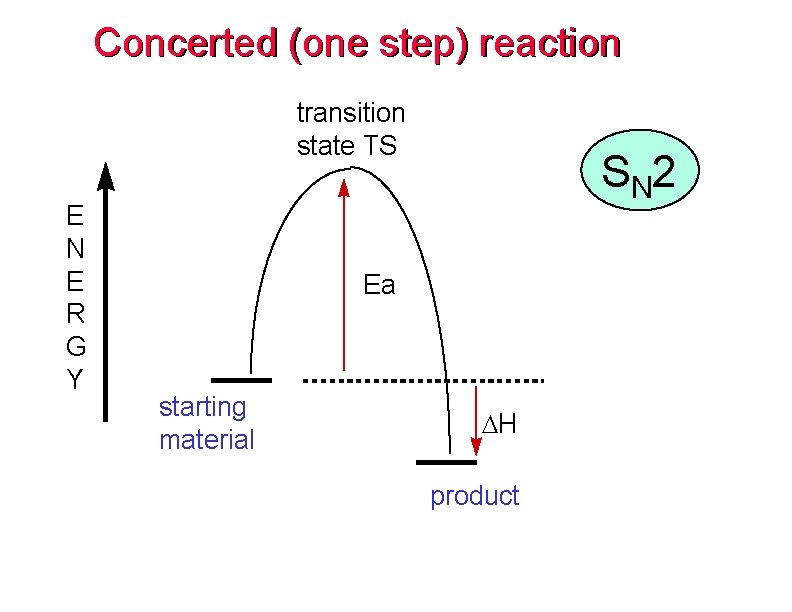
Concerted (one step) reaction transition state TS E N E R G Y S N 2 Ea starting material DH product

REACTION 2 THE SN 1 REACTION
![55 o. C rate = k 1 [RBr] k 1 = 0. 010 liter/mole-sec 55 o. C rate = k 1 [RBr] k 1 = 0. 010 liter/mole-sec](http://slidetodoc.com/presentation_image_h2/72239d303572349bdb616b33283bfdfb/image-18.jpg)
55 o. C rate = k 1 [RBr] k 1 = 0. 010 liter/mole-sec unimolecular two steps slow fast also alkene (via E 1) S N 1 substitution nucleophilic unimolecular
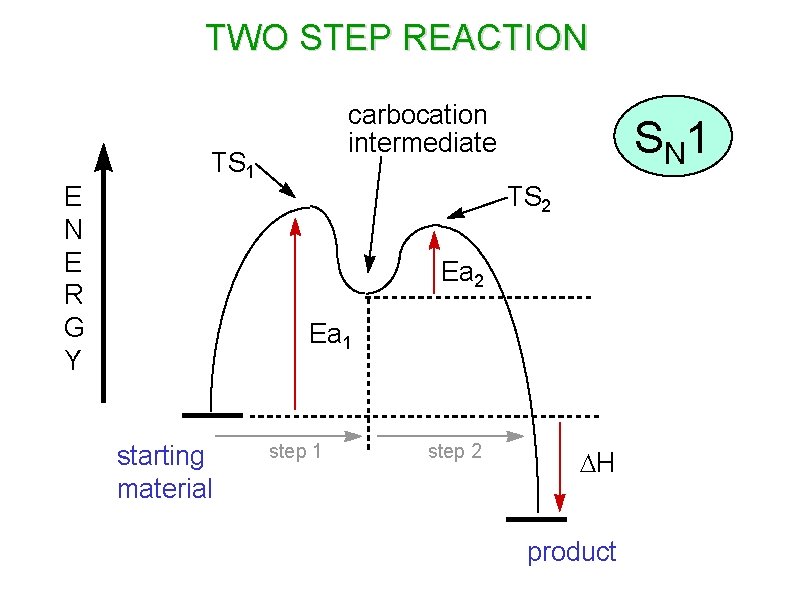
TWO STEP REACTION E R G Y carbocation intermediate TS 1 S N 1 TS 2 Ea 1 starting material step 1 step 2 DH product

A QUICK SUMMARY OF TWO SUBSTITUTION REACTIONS S N 1 / S N 2
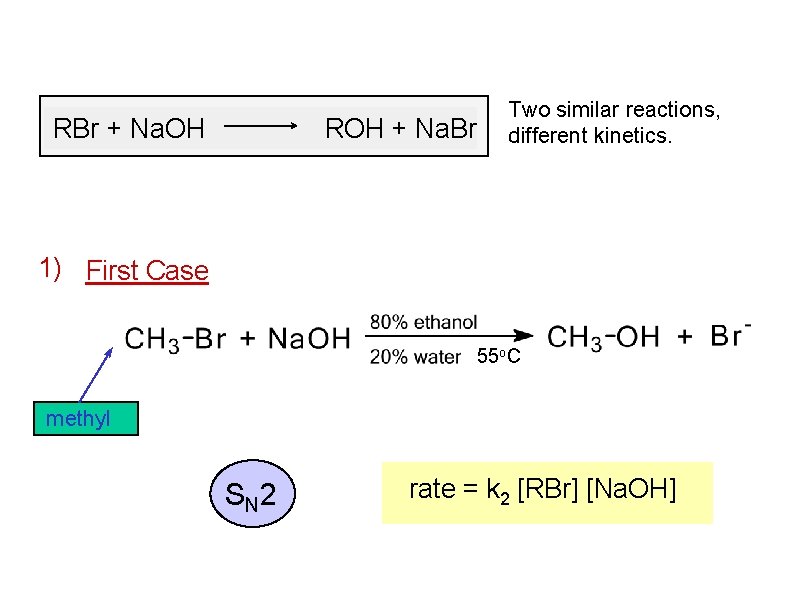
RBr + Na. OH ROH + Na. Br Two similar reactions, different kinetics. 1) First Case 55 o. C methyl S N 2 rate = k 2 [RBr] [Na. OH]
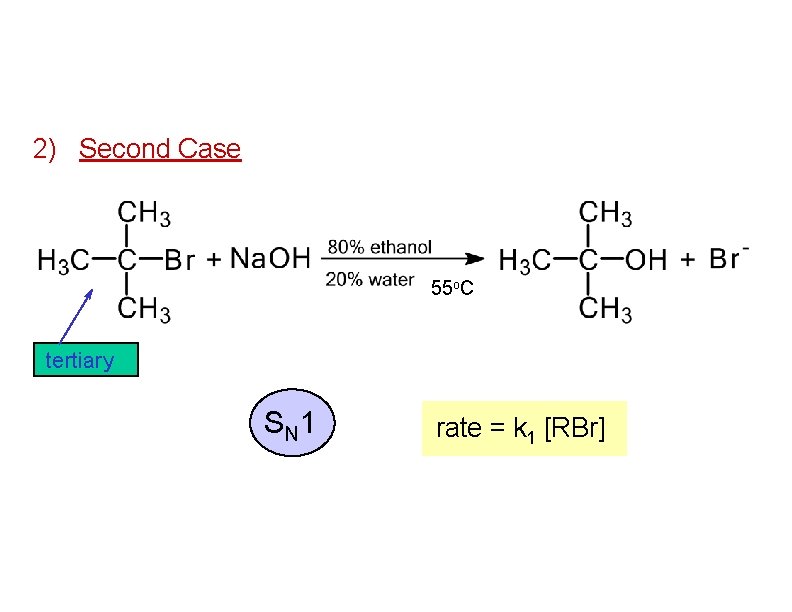
2) Second Case 55 o. C tertiary S N 1 rate = k 1 [RBr]

MANY PARAMETERS INFLUENCE NUCLEOPHILIC SUBSTITUTION
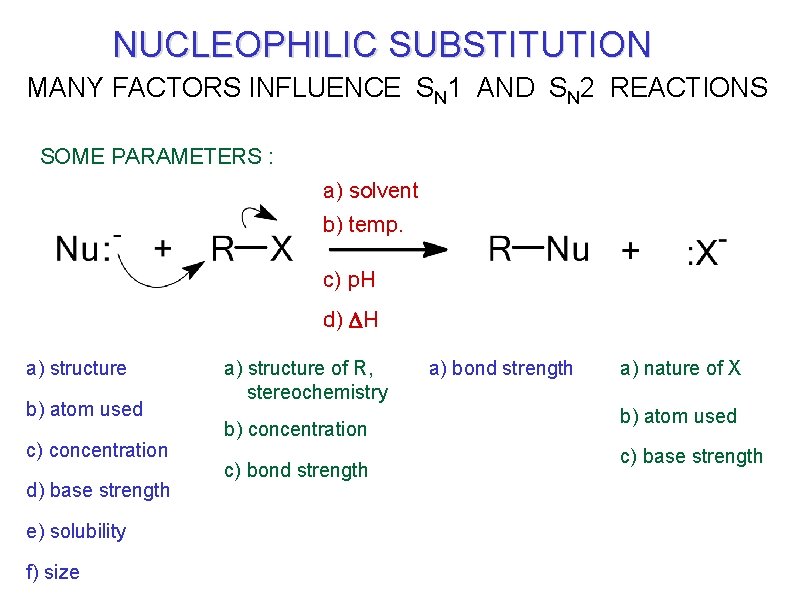
NUCLEOPHILIC SUBSTITUTION MANY FACTORS INFLUENCE SN 1 AND SN 2 REACTIONS SOME PARAMETERS : a) solvent b) temp. c) p. H d) DH a) structure b) atom used c) concentration d) base strength e) solubility f) size a) structure of R, stereochemistry b) concentration c) bond strength a) nature of X b) atom used c) base strength
STRUCTURE OF THE SUBSTRATE S N 1
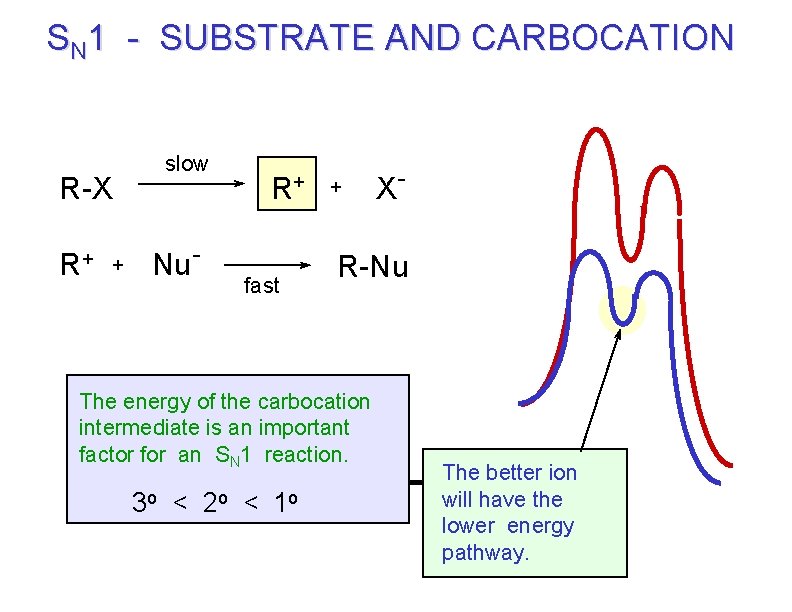
SN 1 - SUBSTRATE AND CARBOCATION R-X R+ + slow Nu- R+ fast + R-Nu The energy of the carbocation intermediate is an important factor for an SN 1 reaction. 3 o < 2 o < 1 o X- The better ion will have the lower energy pathway.
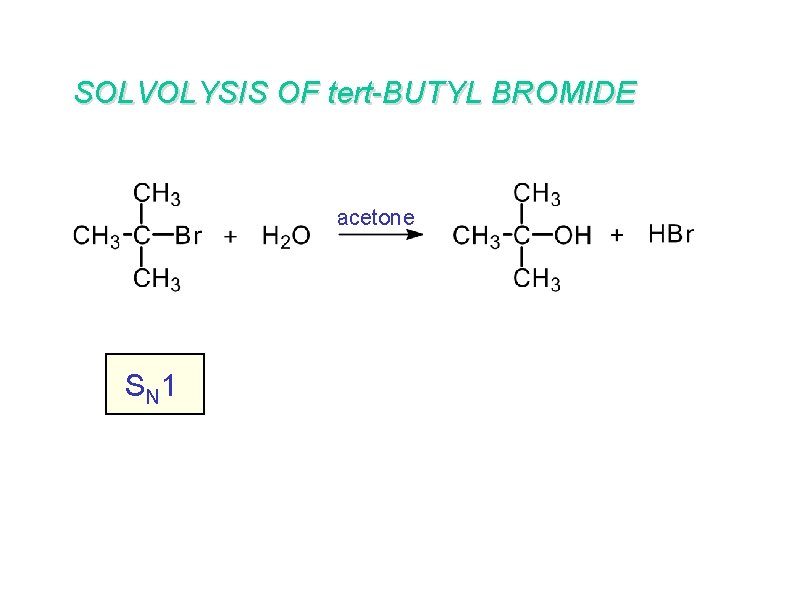
SOLVOLYSIS OF tert-BUTYL BROMIDE acetone S N 1
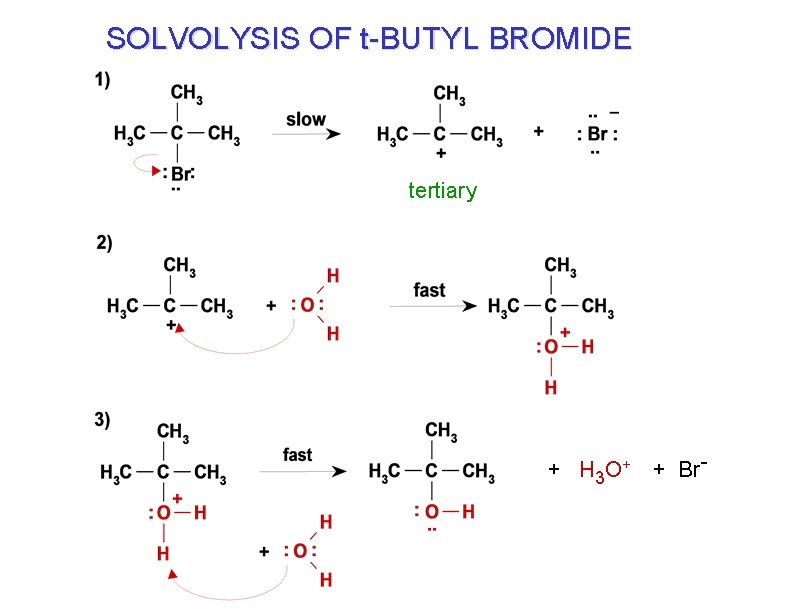
SOLVOLYSIS OF t-BUTYL BROMIDE tertiary + H 3 O + + Br-
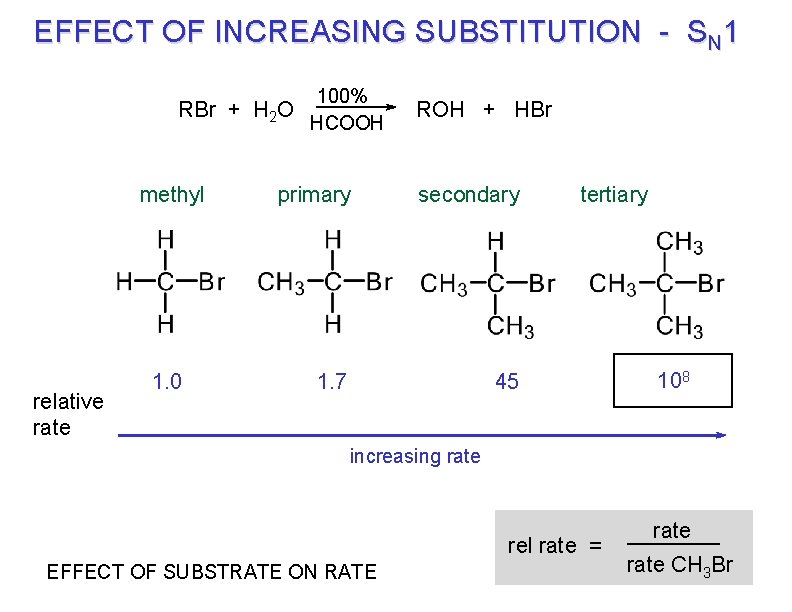
EFFECT OF INCREASING SUBSTITUTION - SN 1 100% RBr + H 2 O HCOOH methyl relative rate 1. 0 ROH + HBr primary secondary 1. 7 45 tertiary 108 ? Guess increasing rate rel rate = EFFECT OF SUBSTRATE ON RATE rate CH 3 Br
STRUCTURE OF THE SUBSTRATE S N 2
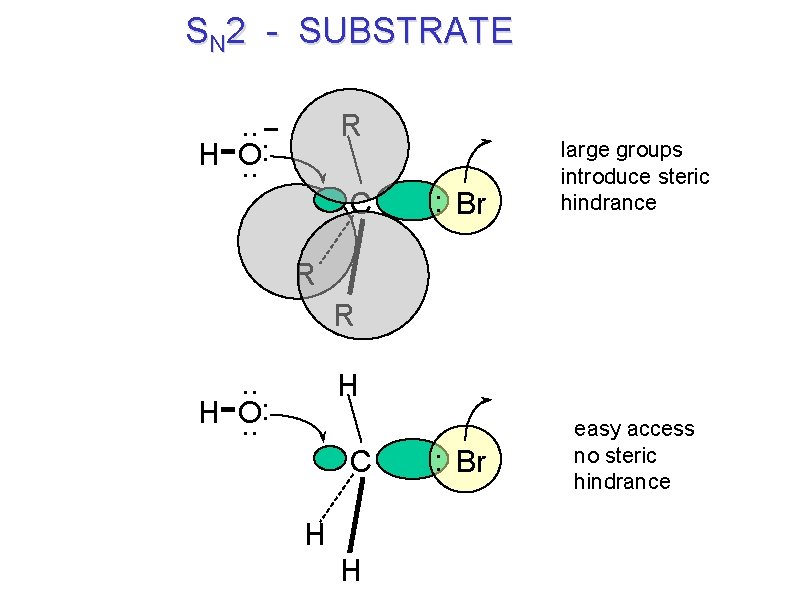
SN 2 - SUBSTRATE. . : H O. . R C : Br large groups introduce steric hindrance R R. . : H O. . H C H H : Br easy access no steric hindrance
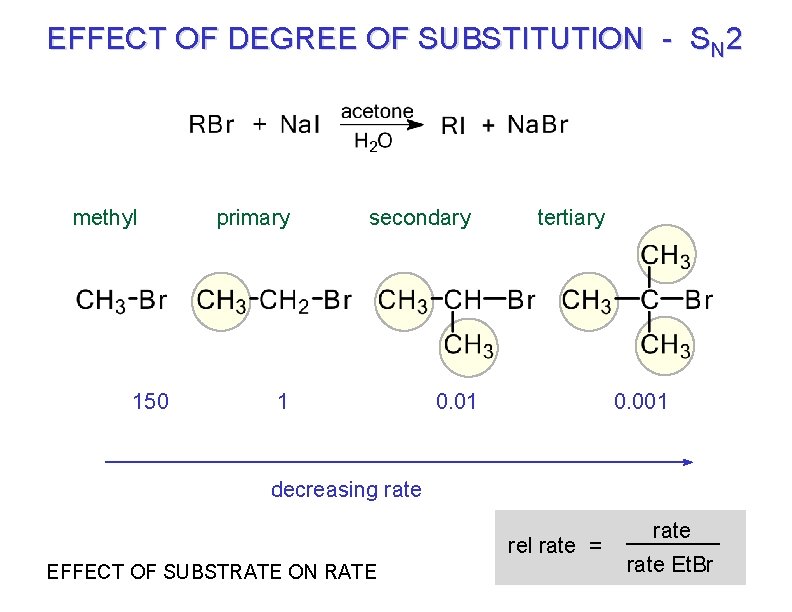
EFFECT OF DEGREE OF SUBSTITUTION - SN 2 methyl 150 primary secondary 1 tertiary 0. 01 0. 001 decreasing rate rel rate = EFFECT OF SUBSTRATE ON RATE rate Et. Br
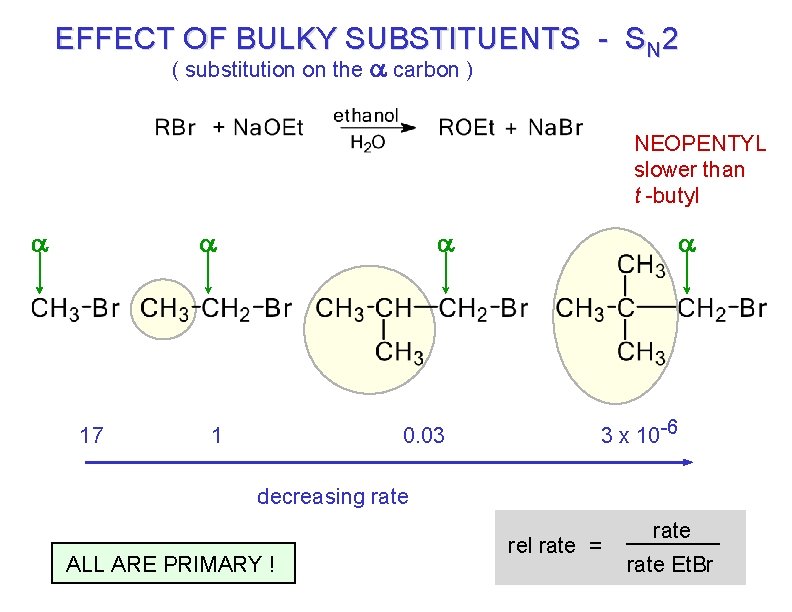
EFFECT OF BULKY SUBSTITUENTS - SN 2 ( substitution on the a carbon ) NEOPENTYL slower than t -butyl a a 17 a 1 0. 03 a 3 x 10 -6 decreasing rate ALL ARE PRIMARY ! rel rate = rate Et. Br
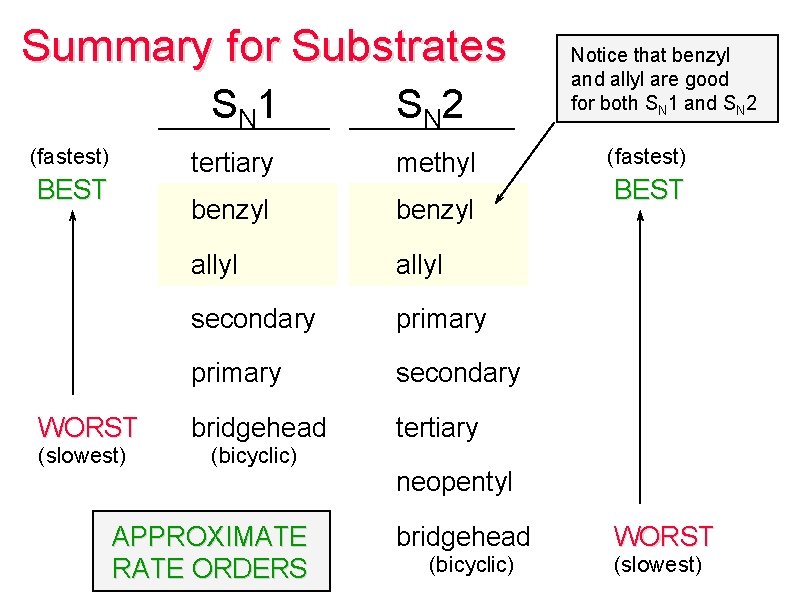
Summary for Substrates S N 1 (fastest) BEST WORST (slowest) S N 2 tertiary methyl benzyl allyl secondary primary secondary bridgehead tertiary (bicyclic) APPROXIMATE RATE ORDERS Notice that benzyl and allyl are good for both SN 1 and SN 2 (fastest) BEST neopentyl bridgehead (bicyclic) WORST (slowest)

IS THE NUCLEOPHILE IMPORTANT IN BOTH SN 1 AND SN 2 REACTIONS ?
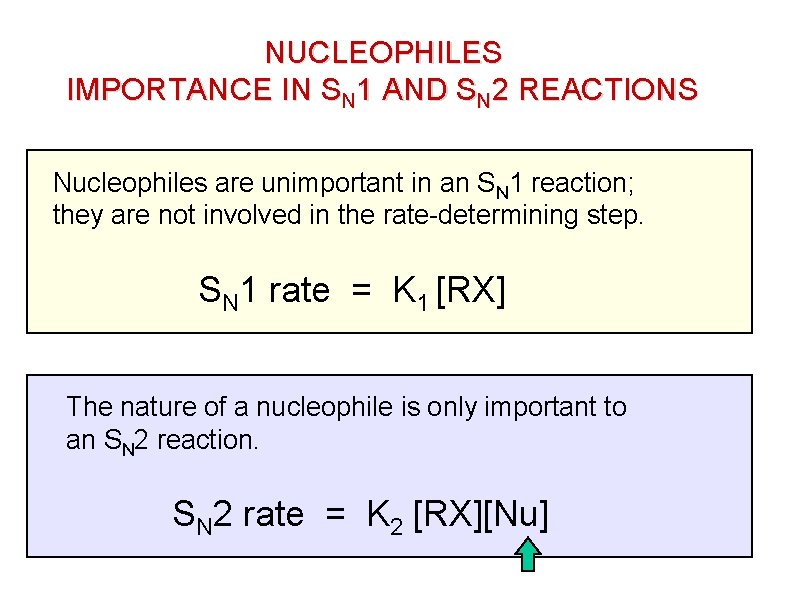
NUCLEOPHILES IMPORTANCE IN SN 1 AND SN 2 REACTIONS Nucleophiles are unimportant in an SN 1 reaction; they are not involved in the rate-determining step. SN 1 rate = K 1 [RX] The nature of a nucleophile is only important to an SN 2 reaction. SN 2 rate = K 2 [RX][Nu]

WHAT IS A GOOD NUCLEOPHILE ? SN 2 REACTIONS
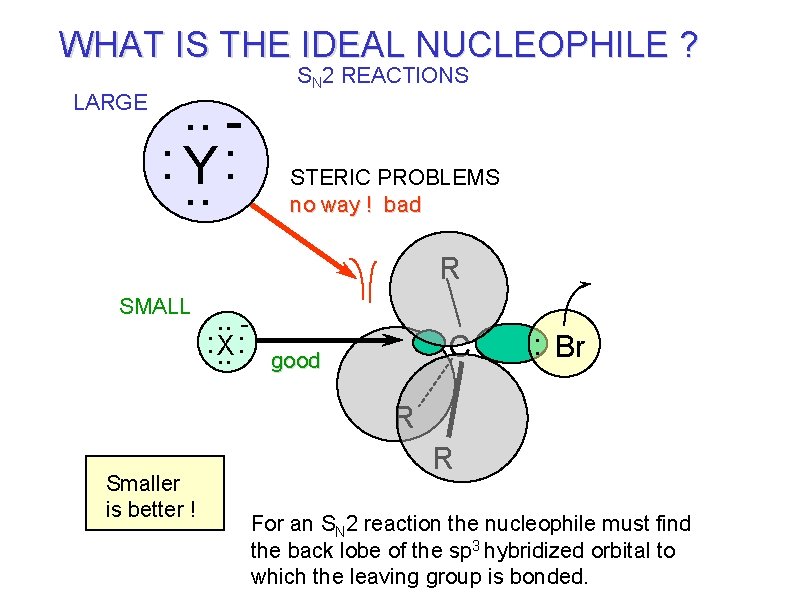
WHAT IS THE IDEAL NUCLEOPHILE ? LARGE . . : . . Y: SN 2 REACTIONS STERIC PROBLEMS no way ! bad R SMALL . . : . . X: C good : Br R Smaller is better ! R For an SN 2 reaction the nucleophile must find the back lobe of the sp 3 hybridized orbital to which the leaving group is bonded.
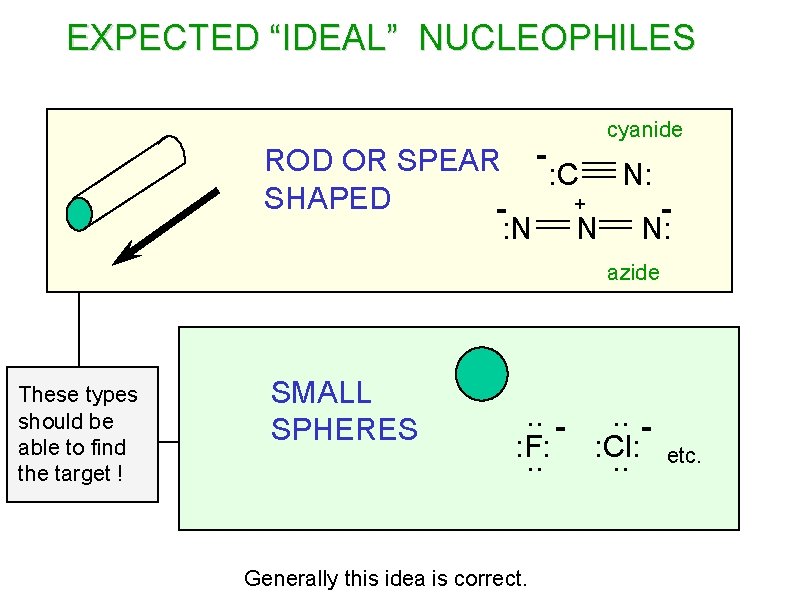
EXPECTED “IDEAL” NUCLEOPHILES cyanide ROD OR SPEAR - : C N: SHAPED + : N N N: azide These types should be able to find the target ! SMALL SPHERES . . : F: : Cl: . . Generally this idea is correct. etc.
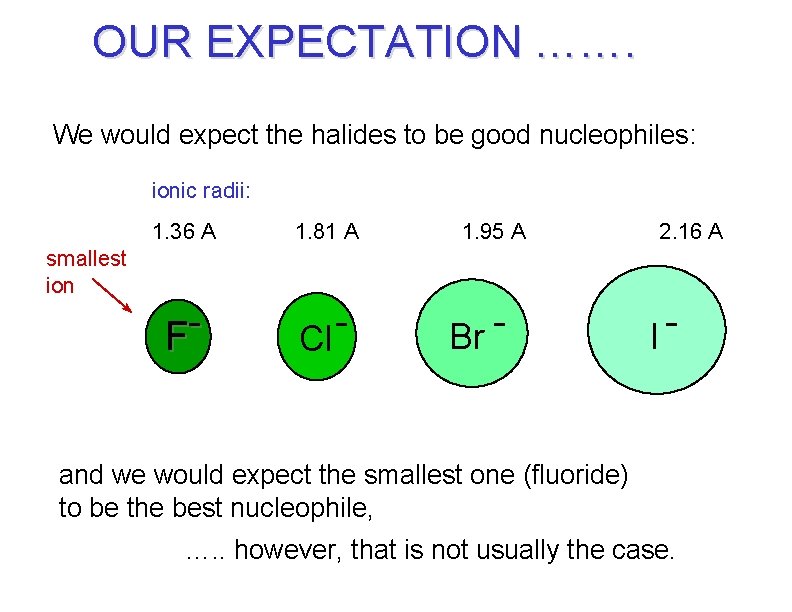
OUR EXPECTATION ……. We would expect the halides to be good nucleophiles: ionic radii: 1. 36 A 1. 81 A F Cl 1. 95 A 2. 16 A smallest ion Br I and we would expect the smallest one (fluoride) to be the best nucleophile, …. . however, that is not usually the case.
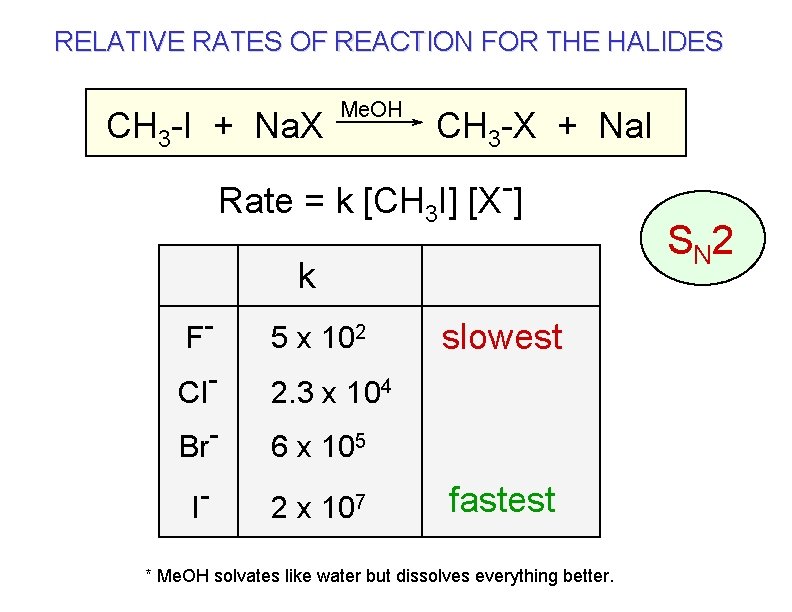
RELATIVE RATES OF REACTION FOR THE HALIDES CH 3 -I + Na. X Me. OH CH 3 -X + Na. I Rate = k [CH 3 I] [X ] k F 5 x 102 Cl. Br 2. 3 x 104 I- slowest 6 x 105 2 x 107 fastest * Me. OH solvates like water but dissolves everything better. S N 2

COMPETITIVE NUCLEOPHILES
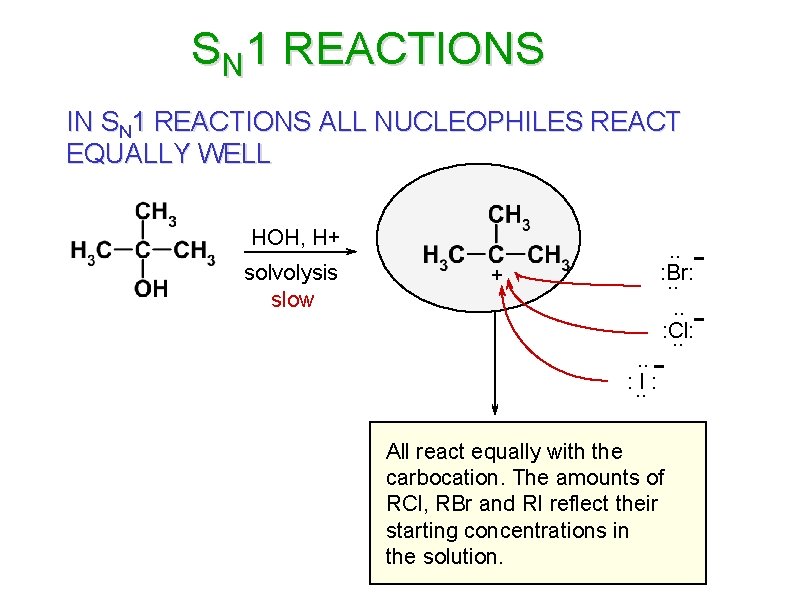
SN 1 REACTIONS IN SN 1 REACTIONS ALL NUCLEOPHILES REACT EQUALLY WELL HOH, H+ solvolysis slow - + . . : Br: . . : Cl: . . - - . . : . . I : All react equally with the carbocation. The amounts of RCl, RBr and RI reflect their starting concentrations in the solution.
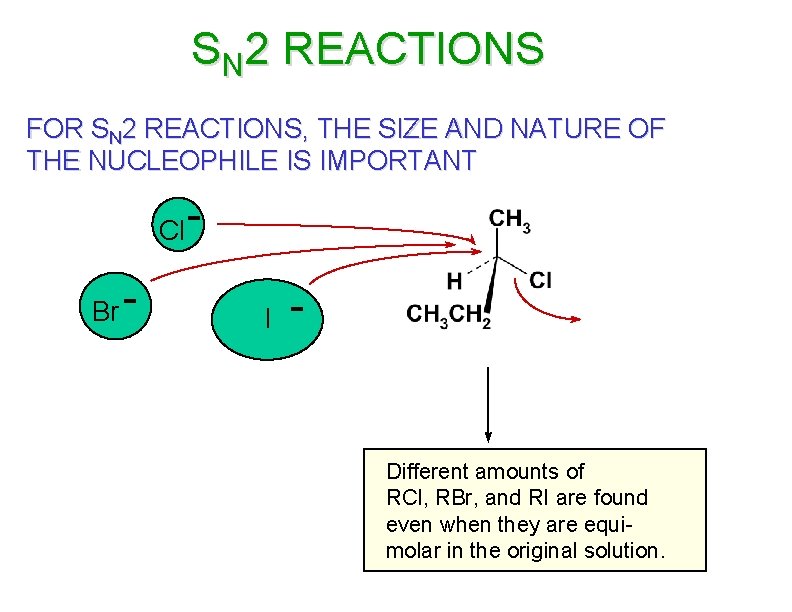
SN 2 REACTIONS FOR SN 2 REACTIONS, THE SIZE AND NATURE OF THE NUCLEOPHILE IS IMPORTANT Cl Br - I - Different amounts of RCl, RBr, and RI are found even when they are equimolar in the original solution.
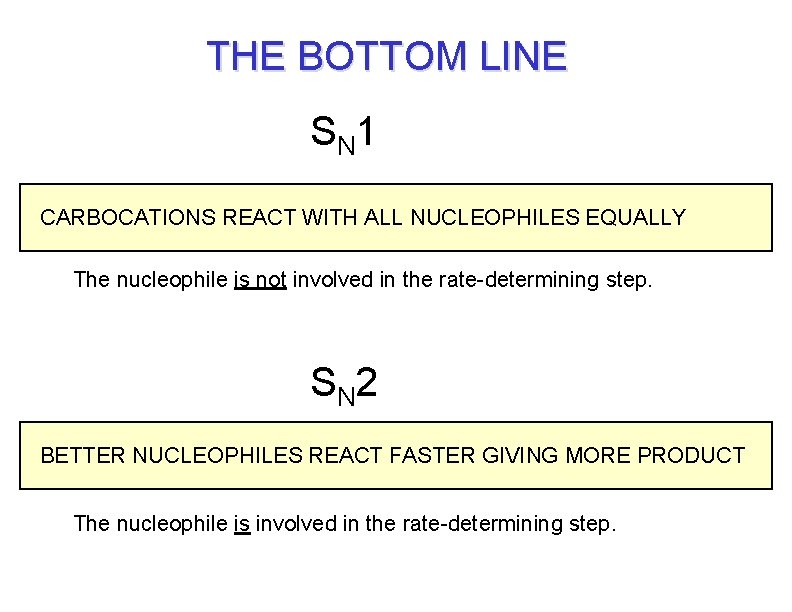
THE BOTTOM LINE S N 1 CARBOCATIONS REACT WITH ALL NUCLEOPHILES EQUALLY The nucleophile is not involved in the rate-determining step. S N 2 BETTER NUCLEOPHILES REACT FASTER GIVING MORE PRODUCT The nucleophile is involved in the rate-determining step.

HOW CAN YOU TELL IF IT IS SN 1 OR SN 2 ? 1) LOOK FIRST AT THE NUCLEOPHILE You cannot do a reasonable SN 2 reaction without a good Nu. If you have a poor nucleophile, SN 1 is probable. 2) NEXT CHECK THE SUBSTRATE Primary is bad for SN 1 …. . Tertiary is bad for SN 2 3) FINALLY LOOK AT THE SOLVENT SN 2 is best in nonpolar and polar aprotic solvents, but can also happen in water or Et. OH. SN 1 needs a polar solvent.
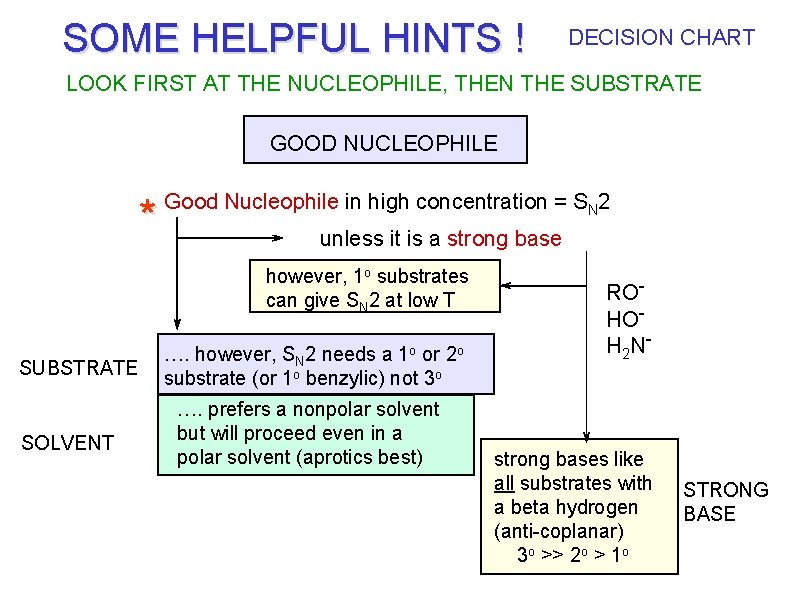
SOME HELPFUL HINTS ! DECISION CHART LOOK FIRST AT THE NUCLEOPHILE, THEN THE SUBSTRATE GOOD NUCLEOPHILE * Good Nucleophile in high concentration = SN 2 unless it is a strong base however, 1 o substrates can give SN 2 at low T SUBSTRATE SOLVENT …. however, SN 2 needs a 1 o or 2 o substrate (or 1 o benzylic) not 3 o …. prefers a nonpolar solvent but will proceed even in a polar solvent (aprotics best) ROHOH 2 N - strong bases like all substrates with a beta hydrogen (anti-coplanar) 3 o >> 2 o > 1 o STRONG BASE
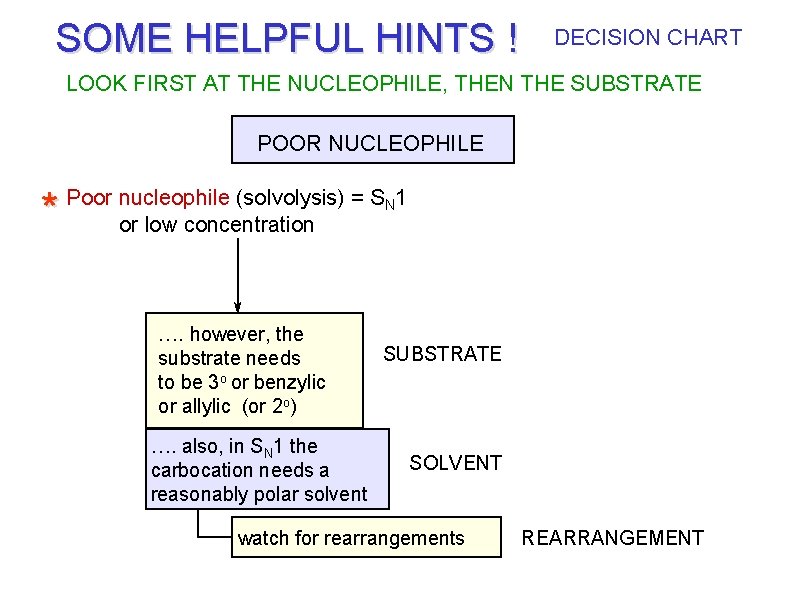
SOME HELPFUL HINTS ! DECISION CHART LOOK FIRST AT THE NUCLEOPHILE, THEN THE SUBSTRATE POOR NUCLEOPHILE * Poor nucleophile (solvolysis) = SN 1 or low concentration …. however, the substrate needs to be 3 o or benzylic or allylic (or 2 o) …. also, in SN 1 the carbocation needs a reasonably polar solvent SUBSTRATE SOLVENT watch for rearrangements REARRANGEMENT

Summary of material you MUST know for the HL Chemistry exam

IB Style Rxn Pathways • Haloalkanes are the starting reactants for all substitution reactions • SN 1 uses slow heterolytic cleavage of the carbon – halogen bond to form a carbocation intermediate as the rate limiting step • The intermediate then reacts rapidly with a nucleophile (Nu-) to form the final product • The energy profile for an SN 1 reaction shows two humps in the reaction pathway (see slide) • You MUST USE CURLY ARROWS to show this process for IB exam credit!
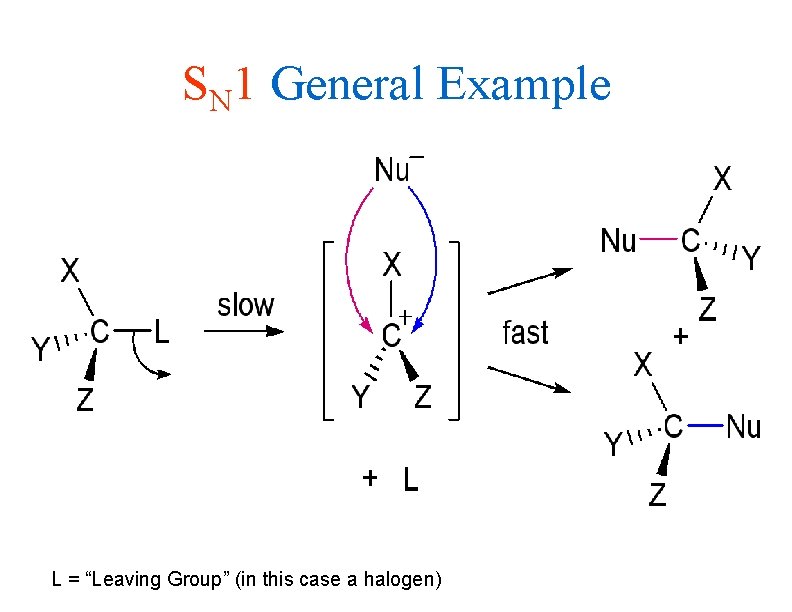
SN 1 General Example L = “Leaving Group” (in this case a halogen)
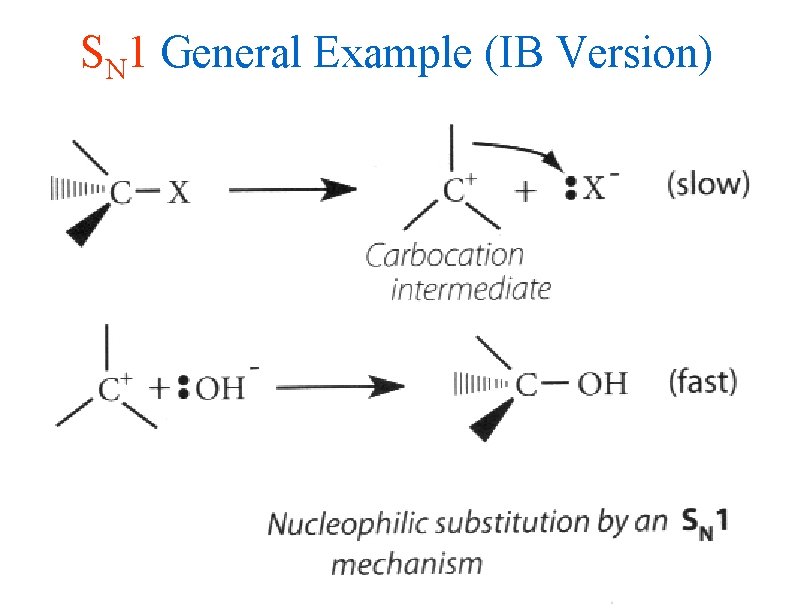
SN 1 General Example (IB Version)
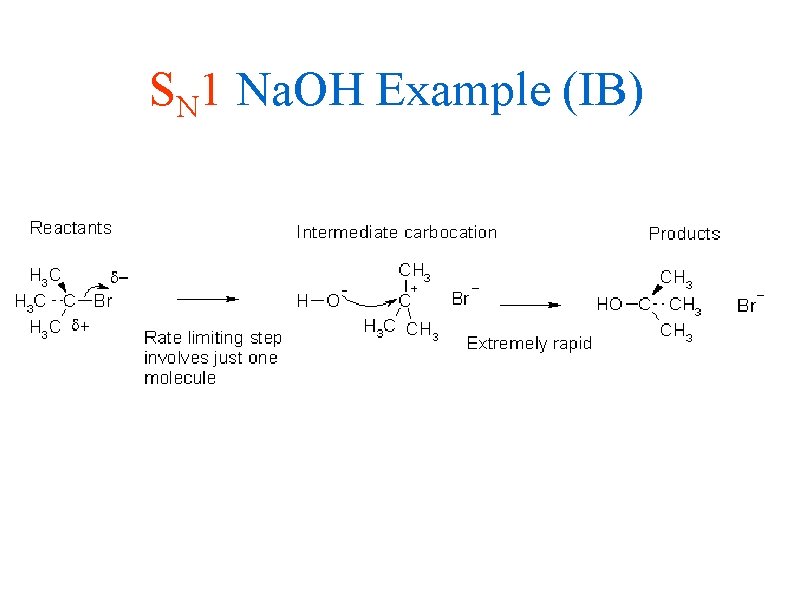
SN 1 Na. OH Example (IB)
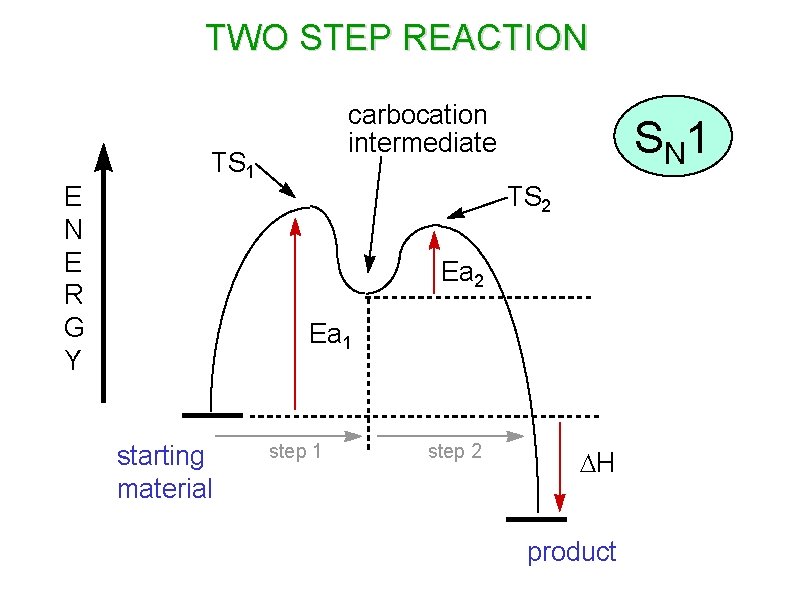
TWO STEP REACTION E R G Y carbocation intermediate TS 1 S N 1 TS 2 Ea 1 starting material step 1 step 2 DH product

IB Style Rxn Pathways • The SN 2 reaction involves the simultaneous breaking of the carbon-halogen bond and the formation of the carbon-nucleophile bond • The transition state has a 5 bonded metastable carbon complex which releases the halogen while the covalent bond to the nucleophile remains • Again, you must be able to use CURLY ARROWS to write out this mechanism
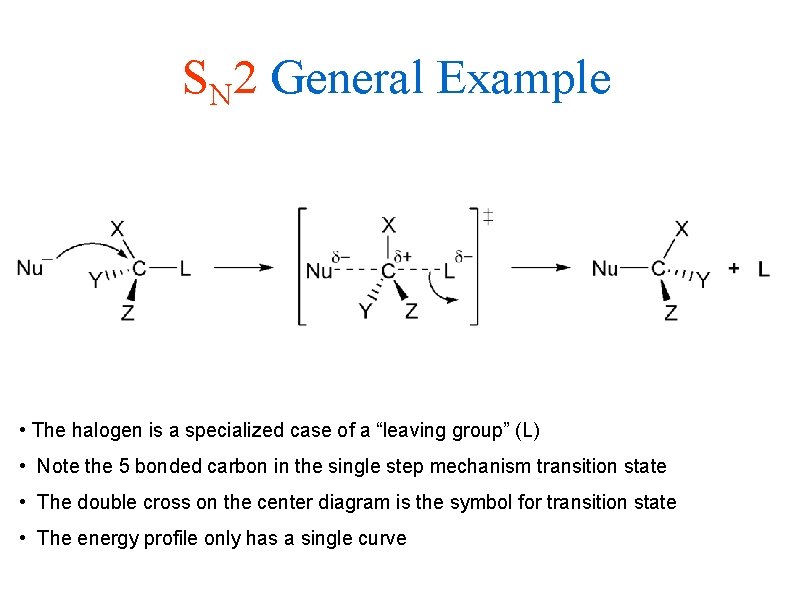
SN 2 General Example • The halogen is a specialized case of a “leaving group” (L) • Note the 5 bonded carbon in the single step mechanism transition state • The double cross on the center diagram is the symbol for transition state • The energy profile only has a single curve
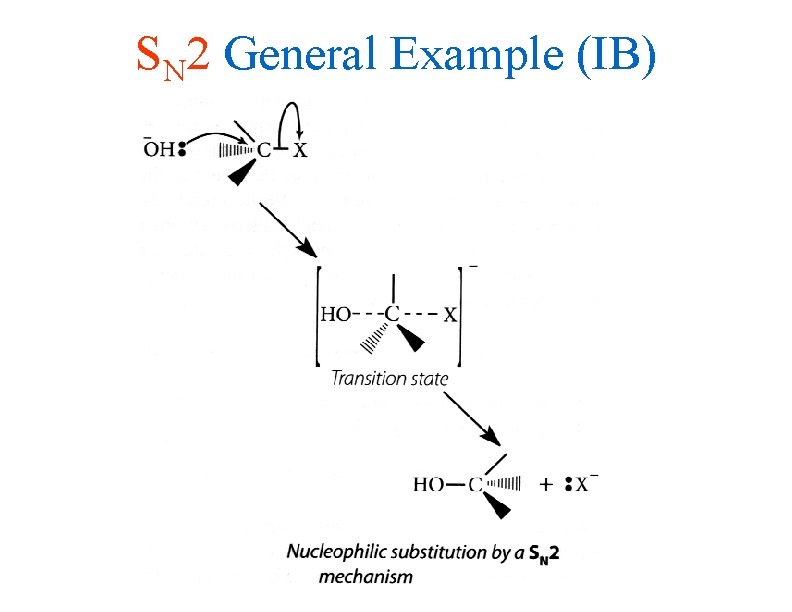
SN 2 General Example (IB)
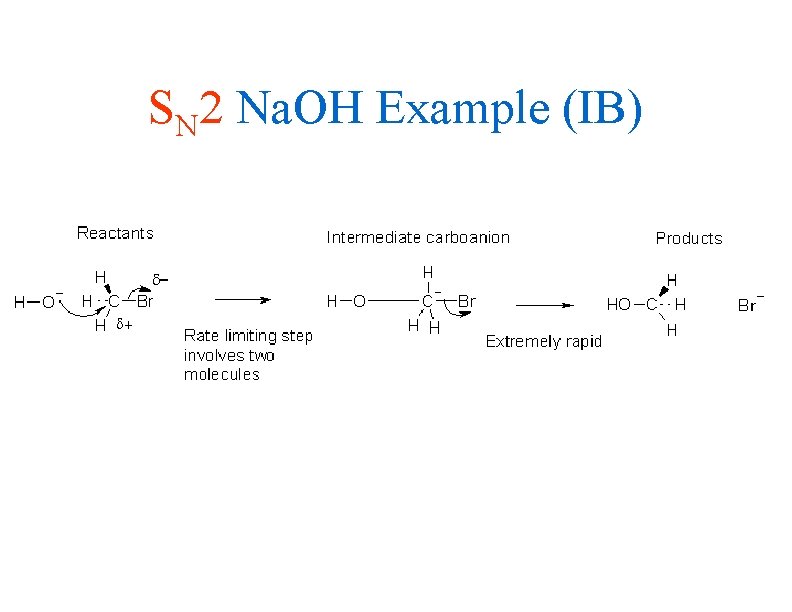
SN 2 Na. OH Example (IB)
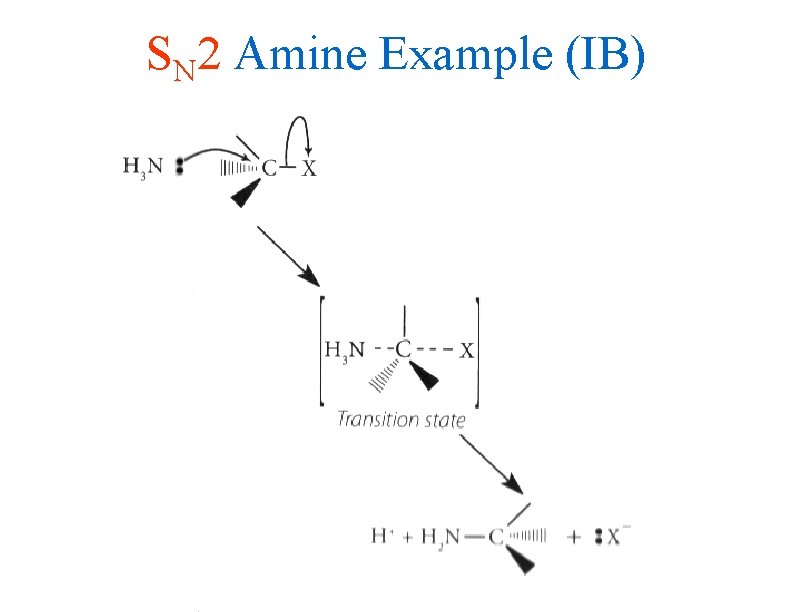
SN 2 Amine Example (IB)
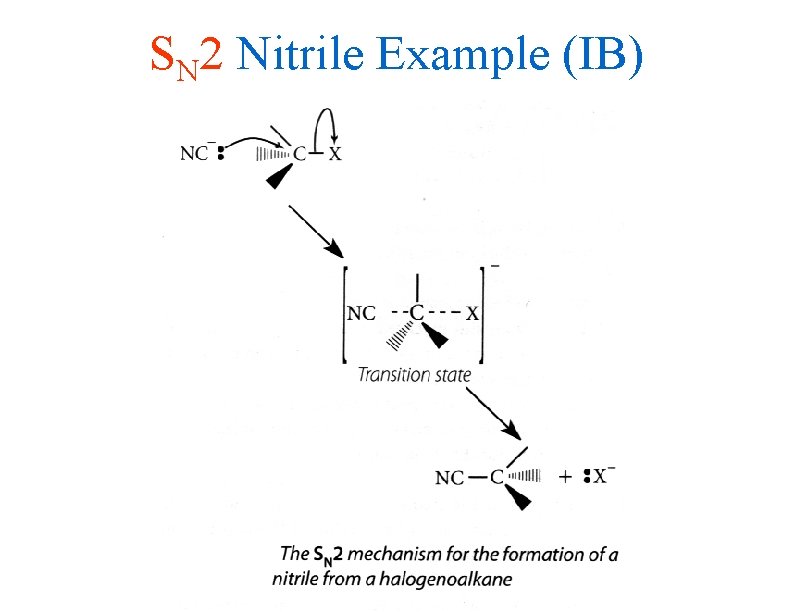
SN 2 Nitrile Example (IB)
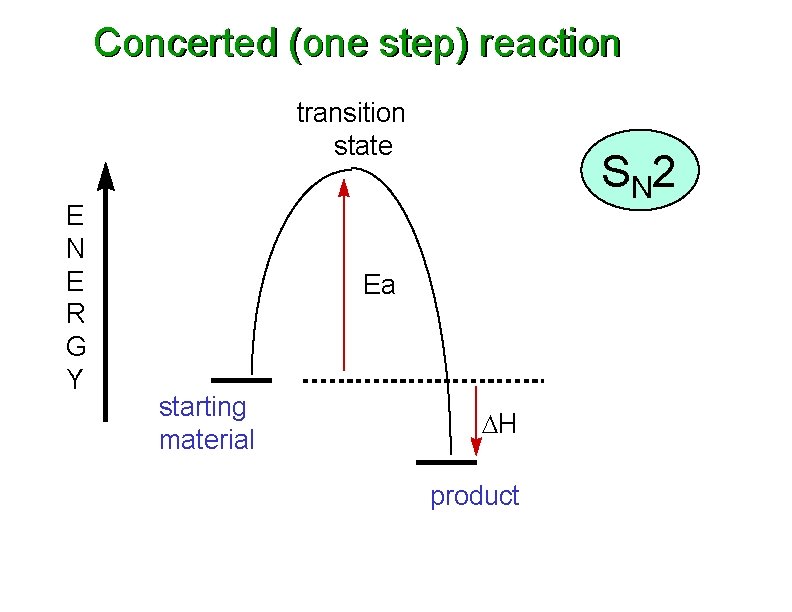
Concerted (one step) reaction transition state E N E R G Y S N 2 Ea starting material DH product
Summary of IB SN Information (1) • Slightly positive charged carbons are attacked by negative nuclophiles • Primary halogenoalkanes react via SN 2 Rate = k[R-X][OH] • Tertiary halogenoalkanes react via SN 1 Rate = k[R-X] • Secondary halogenoalkanes can react via either route, and will probably not be asked on the DP exam
Summary of IB SN Information (2) • As the halogen changes from Cl→ Br→ I, carbon-halogen bond polarity decreases, whereas reaction rate increases • SN 1 reactions occur faster than SN 2 • The rate of reaction for halogenoalkanes is: Tertiary > Secondary > Primary • Nitriles can be converted into: (1) amines using hydrogen gas (with a nickel catalyst) or (2) carboxylic acids using an acid (typically HCl)
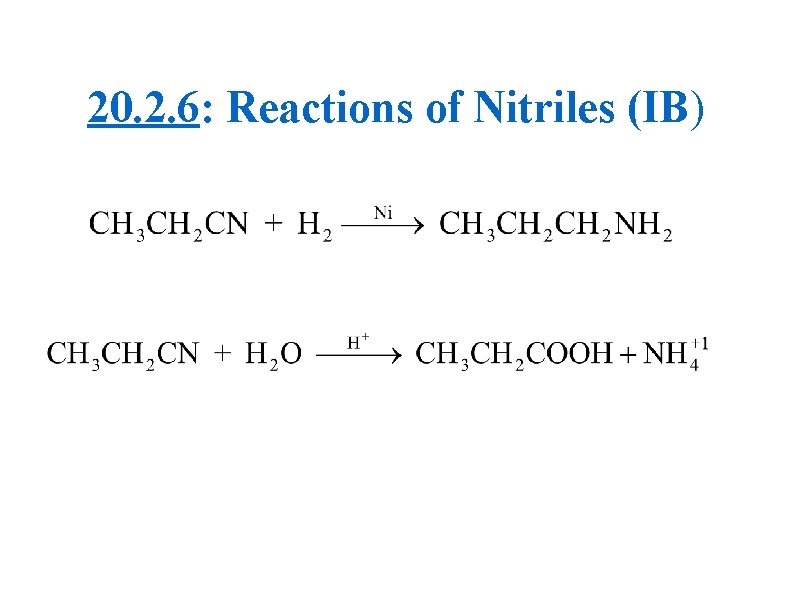
20. 2. 6: Reactions of Nitriles (IB)
Words to the Wise • You have just seen the minimum you need to know about Nucleophilic Substitution Reactions in this mini-review • Read your IB Text Book and review material.
- Slides: 65