HL 7 FHIR Connectathon 20 Day 1 Saturday

























- Slides: 37

HL 7 FHIR Connectathon 20 Day 1 Saturday, January 12, 2019 C-CDA Implementation-A-Thon Track San Antonio © 2017 Health Level Seven ® International. All Rights Reserved. HL 7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U. S. TM Office. 1

Introductions Dave Hamill, HL 7 Lisa Nelson, Track Lead Ø Ø Lnelson@Max. md Jean Duteau, Topic Facilitator Ø Jean@duteaudesign. com Joginder Madra, Collaboration Facilitator Ø Joginder. madra@madraconsulting. com © 2017 Health Level Seven ® International. All Rights Reserved. HL 7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U. S. TM Office. 2 DHamill@hl 7. org

Resources n Confluence Ø Ø n Zulip Ø Ø n HL 7 C-CDA IAT 20190112 -13 Google Drive Ø n https: //chat. FHIR. org Use the gear tool by STREAMS to subscribe to the C-CDA IAT Stream Drop. Box Ø n https: //confluence. hl 7. org Resources Page: https: //confluence. hl 7. org/display/IAT/Resources HL 7 C-CDA IAT 20190112 -13 CDA Examples Task Force Ø http: //hl 7 -c-cda-examples. herokuapp. com/ © 2017 Health Level Seven ® International. All Rights Reserved. HL 7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U. S. TM Office. 3

January 12 th Agenda n Saturday Q 1 Encounter Summary Documents vs Patient Summary Documents Utilize guidance developed in the Commonwell/Carequality IG. Participants will bring prepared C-CDA sample documents and their questions. Documents will be exchanged and reviewed for consistency and consumption capability. Q 2 USCDI Utilize guidance available from ONC, maturity ranking for each data class developed at the ONC Interoperability Forum. Participants will bring C-CDA documents that demonstrate representation of selected USCDI data classes. Participants will discuss areas where guidance is not clear enough to guide consistent representation, identify opportunities for greater consistency, and generate examples to be considered by the CDA Examples Task Force to improve the pool of verified examples available to implementers. Q 3 Clinical Notes Demonstrate how to use new C-CDA supplemental templates for Notes Section and Note Activity. Participants will bring C-CDA documents that demonstrate representation of clinical note information. Participants will discuss areas where guidance is not clear enough to enable consistent representation, identify opportunities for greater consistency, and generate examples to be considered by the CDA Examples Task Force to improve the pool of verified examples available to implementers. Q 4 Handle C-CDA documents within FHIR APIs Participants will explore options for exchanging C-CDA documents via FHIR APIs. Participants will use conversion mechanisms to experiment with converting C-CDA documents into FHIR C-CDA documents, and converting FHIR documents to C-CDA documents. © 2017 Health Level Seven ® International. All Rights Reserved. HL 7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U. S. TM Office. 4

Encounter Summary Documents vs Patient Summary Documents Utilize guidance developed in the Commonwell/Carequality IG. Participants will bring prepared C-CDA sample documents and their questions. Documents will be exchanged and reviewed for consistency and consumption capability. SATURDAY, Q 1 © 2017 Health Level Seven ® International. All Rights Reserved. HL 7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U. S. TM Office. 5

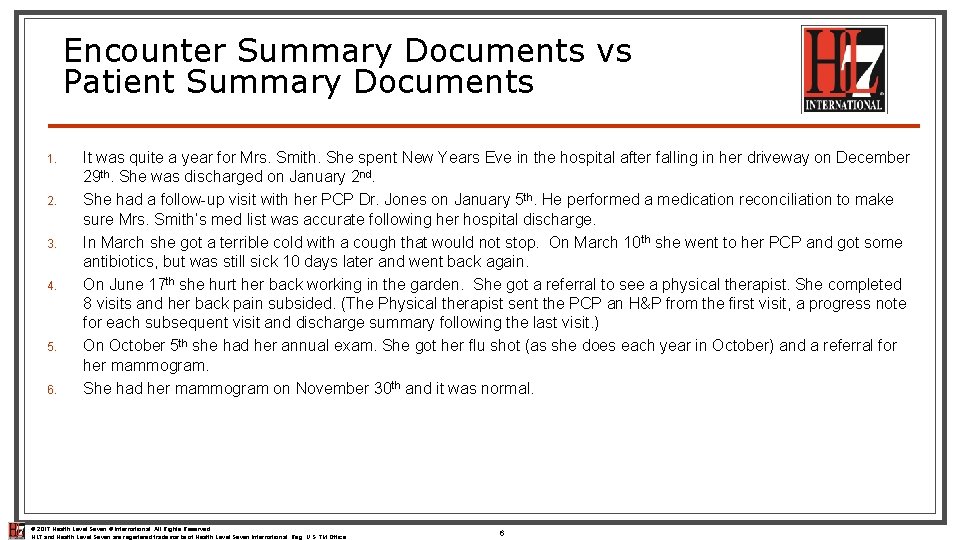
Encounter Summary Documents vs Patient Summary Documents 1. 2. 3. 4. 5. 6. It was quite a year for Mrs. Smith. She spent New Years Eve in the hospital after falling in her driveway on December 29 th. She was discharged on January 2 nd. She had a follow-up visit with her PCP Dr. Jones on January 5 th. He performed a medication reconciliation to make sure Mrs. Smith’s med list was accurate following her hospital discharge. In March she got a terrible cold with a cough that would not stop. On March 10 th she went to her PCP and got some antibiotics, but was still sick 10 days later and went back again. On June 17 th she hurt her back working in the garden. She got a referral to see a physical therapist. She completed 8 visits and her back pain subsided. (The Physical therapist sent the PCP an H&P from the first visit, a progress note for each subsequent visit and discharge summary following the last visit. ) On October 5 th she had her annual exam. She got her flu shot (as she does each year in October) and a referral for her mammogram. She had her mammogram on November 30 th and it was normal. © 2017 Health Level Seven ® International. All Rights Reserved. HL 7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U. S. TM Office. 6

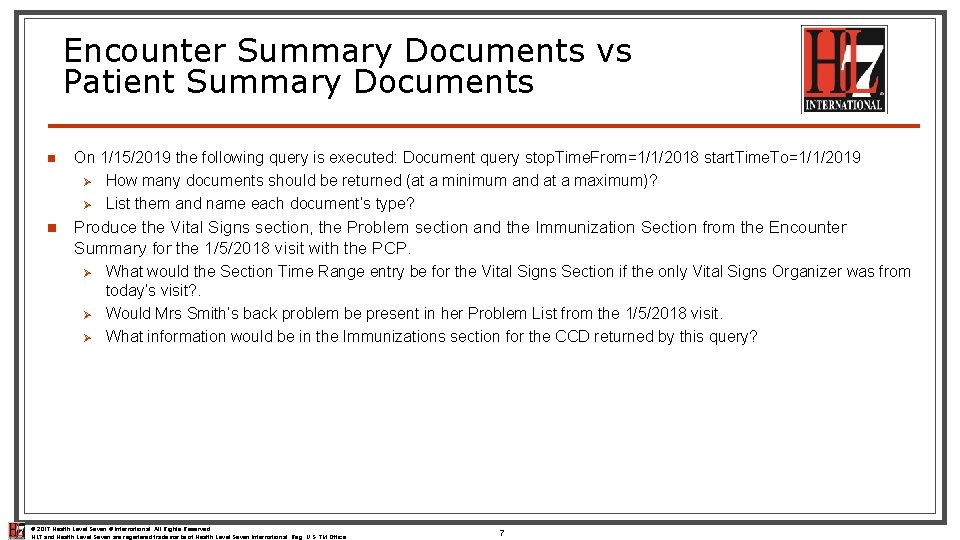
Encounter Summary Documents vs Patient Summary Documents n On 1/15/2019 the following query is executed: Document query stop. Time. From=1/1/2018 start. Time. To=1/1/2019 Ø How many documents should be returned (at a minimum and at a maximum)? Ø List them and name each document’s type? n Produce the Vital Signs section, the Problem section and the Immunization Section from the Encounter Summary for the 1/5/2018 visit with the PCP. Ø Ø Ø What would the Section Time Range entry be for the Vital Signs Section if the only Vital Signs Organizer was from today’s visit? . Would Mrs Smith’s back problem be present in her Problem List from the 1/5/2018 visit. What information would be in the Immunizations section for the CCD returned by this query? © 2017 Health Level Seven ® International. All Rights Reserved. HL 7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U. S. TM Office. 7

n Your system currently produces CCD documents only. You now need to produce a Progress Note at the end of each visit (or for visit(s) that occurred in a period of time). Ø What do you need to change? © 2017 Health Level Seven ® International. All Rights Reserved. HL 7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U. S. TM Office. 8

n Header Differences: Ø component. Of/encompassing. Encounter n n Ø Ø Ø n responsible. Party Location documentation. Of/service. Event author authenticator/Legal. Authenticator Content Differences © 2017 Health Level Seven ® International. All Rights Reserved. HL 7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U. S. TM Office. 9

USCDI Utilize guidance available from ONC, maturity ranking for each data class developed at the ONC Interoperability Forum. Participants will bring C-CDA documents that demonstrate representation of selected USCDI data classes. Participants will discuss areas where guidance is not clear enough to guide consistent representation, identify opportunities for greater consistency, and generate examples to be considered by the CDA Examples Task Force to improve the pool of verified examples available to implementers. SATURDAY, Q 2 © 2017 Health Level Seven ® International. All Rights Reserved. HL 7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U. S. TM Office. 10

Results (Tests and Test Results) n Lots of variation in use of LOINC vs local codes Ø n What get’s put in the Results Section Ø n n n Missing and non-standard code sets Imaging note in the result, escript messenger refills in result? ? ? Values in reference ranges differ What code goes in the organizer. code? How to code a panel (ie PQH 9)? How to code the question/answer pair? How to do microbiologies in general get recorded? © 2017 Health Level Seven ® International. All Rights Reserved. HL 7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U. S. TM Office. 11

Assessment and Plan One section or two n Assessment vs. Clinical Notes sections/note activity n Documents are not consistent in this area n Ø Would this be more than physical assessments? What goes here? Need more clarity about what goes in this section n Wide range of assessment types n Ø Should “Assessments” be recorded in the Result Section? © 2017 Health Level Seven ® International. All Rights Reserved. HL 7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U. S. TM Office. 12

Medications n n n Compounding, mixtures, a-typical meds Seems like Rx. Norm is the norm, But HEDIS measure use NDC…. Rendering differences, what fields should be presented to the humans? Free text sig Medication Status – How do you know what belongs “on the list” Ø Impact of mood. Code (INT vs EVN) © 2017 Health Level Seven ® International. All Rights Reserved. HL 7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U. S. TM Office. 13

Procedures n Procedures that happened, but may or may not have a result. Ø Ø Ø n n n May have been unsuccessful Should not be considered as “fulfilling a care gap” (example: Newborn hearing screening) Coding: SNOMED CT, CPT-2 needed in translation Make sure it is attributed to a performer Result and Procedure or just Result Ø In the Result, use the Procedure Code for the Result Organizer include the result in the result observation n Try to not duplicate information n Do you link the Procedure to the Result Organizer? Ø We also have the Procedure with it in the Procedure section – even if it is a chem panel? © 2017 Health Level Seven ® International. All Rights Reserved. HL 7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U. S. TM Office. 14

Problems Problem type consistency n How many Problem Observations per Problem Concern n Consistency on use of other templates n © 2017 Health Level Seven ® International. All Rights Reserved. HL 7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U. S. TM Office. 15

Clinical Notes Demonstrate how to use new C-CDA supplemental templates for Notes Section and Note Activity. Participants will bring C-CDA documents that demonstrate representation of clinical note information. Participants will discuss areas where guidance is not clear enough to enable consistent representation, identify opportunities for greater consistency, and generate examples to be considered by the CDA Examples Task Force to improve the pool of verified examples available to implementers. SATURDAY, Q 3 © 2017 Health Level Seven ® International. All Rights Reserved. HL 7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U. S. TM Office. 16

Clinical Notes n Your system collects textual notes recorded by the physician. Add the notes that were added from the visit on June 17 th. Ø Where would you include them in the document? © 2017 Health Level Seven ® International. All Rights Reserved. HL 7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U. S. TM Office. 17

Handle C-CDA documents within FHIR APIs Participants will explore options for exchanging C-CDA documents via FHIR APIs. Participants will use conversion mechanisms to experiment with converting C-CDA documents into FHIR C-CDA documents, and converting FHIR documents to C-CDA documents. SATURDAY, Q 4 © 2017 Health Level Seven ® International. All Rights Reserved. HL 7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U. S. TM Office. 18

Resources n Download the mapping file C-CDA to FHIR Ø n https: //confluence. hl 7. org/display/IAT/Resources Download the mapping file C-CDA to C-CDA on FHIR Ø https: //confluence. hl 7. org/display/SOA/Project+Documents © 2017 Health Level Seven ® International. All Rights Reserved. HL 7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U. S. TM Office. 19

Educational Material n FHIR Interactions Ø n About FHIR Documents Ø n http: //hl 7. org/FHIR/documents. html Structured Documents Ø Ø n http: //hl 7. org/FHIR/overview-clinical. html FHIR Documents Paradigm Ø n http: //hl 7. org/FHIR/overview-dev. html Composition Resource Bundle Resource with type=“document” Unstructured Documents Ø Ø Binary Resource for PDF (or source/original CDA document) Document Reference © 2017 Health Level Seven ® International. All Rights Reserved. HL 7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U. S. TM Office. 20

FHIR Documents FHIR resources can be used to build documents that represent a composition: a coherent set of information that is a statement of healthcare information, including clinical observations and services. A document is an immutable set of resources with a fixed presentation that is authored and/or attested by humans, organizations and devices. n http: //hl 7. org/FHIR/documents. html n © 2017 Health Level Seven ® International. All Rights Reserved. HL 7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U. S. TM Office. 21

Document Content All documents area Bundle with type “document” that has a Composition as its first resource, followed by a series of resources referenced by the Composition. n The resources include both human readable and computer processable information. n The bundle may include CSS stylesheet references n The bundle may include Provenance Statements and a signature. n © 2017 Health Level Seven ® International. All Rights Reserved. HL 7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U. S. TM Office. 22

Composition Resource n The composition resource is the foundation of the clinical document. It: Ø Ø Ø n provides identity and its purpose, and sets the context of the document carries key information such as the subject and author, and who attests to the document divides the document up into a series of sections, each with their own narrative http: //hl 7. org/FHIR/documents. html © 2017 Health Level Seven ® International. All Rights Reserved. HL 7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U. S. TM Office. 23

How to: Do-It-Yourself n n n Step 1: Create a Composition resource Step 2: Ensure the subject, author, and custodian references point to valid Patient, Practitioner, and Organization resources (can create yourself, get from other tracks or query a FHIR server for them). Step 3: POST the Composition to a FHIR server Step 4: Check the operation outcome to ensure it was successful Step 5: Call $document on the Composition (if supported by the server) to get a full document Bundle back, or create the Bundle through other means (custom code, etc. ) © 2017 Health Level Seven ® International. All Rights Reserved. HL 7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U. S. TM Office. 24

How to: Full-Service Get a “test” Direct address n Open a message, attach a C-CDA document n Send to Direct-enabled FHIR API end point n Receive Dispatched message delivery notification (MDN) n Click through viewing the resources that make up the FHIR document n © 2017 Health Level Seven ® International. All Rights Reserved. HL 7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U. S. TM Office. 25

Max. MD Eval Restful API Link to API Documentation n Testing is OK! n © 2017 Health Level Seven ® International. All Rights Reserved. HL 7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U. S. TM Office. 26

How to: Built-in Transform C-CDA document with your application n Transform it manually or with tool n Compare results n Review the mapping documents, identify differences n © 2017 Health Level Seven ® International. All Rights Reserved. HL 7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U. S. TM Office. 27

HL 7 FHIR Connectathon 20 DAY 2 Sunday, January 13, 2019 C-CDA Implementation-A-Thon Track San Antonio © 2017 Health Level Seven ® International. All Rights Reserved. HL 7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U. S. TM Office. 28

January 13 th Agenda n Sunday Q 1 Care Plan Documents Participants will bring C-CDA Care Plan documents that demonstrate representation of care plan information. Care Plan documents may include a Document Summary Section as specified in the IHE DSS profile. Participants will discuss areas where guidance is not clear enough to guide consistent representation, identify opportunities for greater consistency, and generate examples to be considered by the CDA Examples Task Force to improve the pool of verified examples available to implementers. Q 2 Provenance Participants will discuss issues related to defining and representing data provenance in C-CDA documents. Q 3 Scorecard Participants will learn and provide feedback on the rules developed for the Scorecard tool and for plans developed around the CDA roadmap. Participants will discuss and provide input on approaches to change management and release scheduling that support implementer preferences and recognize implementer challenges. Participants will provide feedback on uptake for new versions of C-CDA Templates: Considerations for errata releases, Value Set updates and possible C-CDA R 2. 2 Q 4 Emergent Topics Participants will conduct additional discussions for parking lot items. Optionally, participants may discuss emerging topics such as the representing Social Determinants of Health. Participants interested in exploring other FHIR tracks may join with other track programs. © 2017 Health Level Seven ® International. All Rights Reserved. HL 7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U. S. TM Office. 29

Care Plan Documents Participants will bring C-CDA Care Plan documents that demonstrate representation of care plan information. Care Plan documents may include a Document Summary Section as specified in the IHE DSS profile. Participants will discuss areas where guidance is not clear enough to guide consistent representation, identify opportunities for greater consistency, and generate examples to be considered by the CDA Examples Task Force to improve the pool of verified examples available to implementers. SUNDAY, Q 1 © 2017 Health Level Seven ® International. All Rights Reserved. HL 7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U. S. TM Office. 30

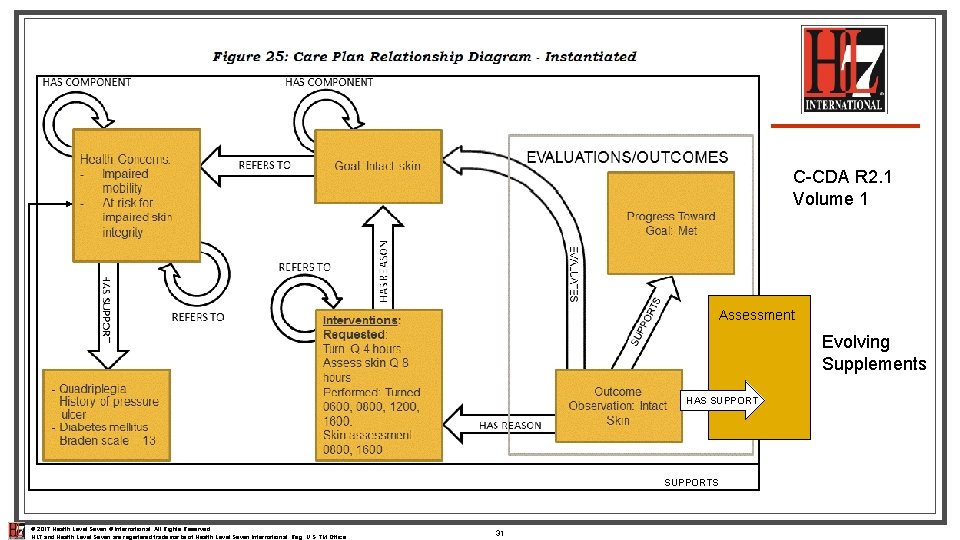
C-CDA R 2. 1 Volume 1 Assessment Evolving Supplements HAS SUPPORTS © 2017 Health Level Seven ® International. All Rights Reserved. HL 7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U. S. TM Office. 31

Care Plan Document Common understanding/agreement about how to represent content for 3 cornerstone sections n Update on the emerging view on Assessment information within the Health Status Evaluations and Outcomes Section n Common understanding/agreement about what type of linkages are defined from one entry type to another n Introduction to the notion of a “document summary section” n © 2017 Health Level Seven ® International. All Rights Reserved. HL 7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U. S. TM Office. 32

Care Plan Documents n Discussion Points Ø Ø Ø Provide links to access the IHE guidance - DSS Document Summary Section Gather feedback from Care Plan document implementers to learn what would be most helpful to them in the IAT session How to see the variances in what people are implementing, see potential examples on a section by section basis Discuss goal of gathering examples that could go to CDA Examples task force and become referenced examples Go over materials and assignment/expectations - get commitment for who will be contributing what Review expectation for all documents to be posted before Jan 9 th. © 2017 Health Level Seven ® International. All Rights Reserved. HL 7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U. S. TM Office. 33

Provenance Participants will discuss issues related to defining and representing data provenance in CCDA documents. SUNDAY, Q 2 © 2017 Health Level Seven ® International. All Rights Reserved. HL 7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U. S. TM Office. 34

Wednesday, December 5 th n Prep for Provenance Group Discussion Ø Ø Ø n n n Review prior discussion notes on Data Provenance from Sept HL 7 WG meeting and emerging guidance. Discuss how to structure the discussions to be most useful at CDA IAT. Discuss options for contributing samples that could be reviewed and provided to CDA Examples Task Force Establish a shared definition What is required? What are the use cases – generate a list? How do we do this already? How does provenance carry forward? Go through the header and entry data elements, what about sections? © 2017 Health Level Seven ® International. All Rights Reserved. HL 7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U. S. TM Office. 35

Scorecard Participants will learn and provide feedback on the rules developed for the Scorecard tool and for plans developed around the CDA roadmap. Participants will discuss and provide input on approaches to change management and release scheduling that support implementer preferences and recognize implementer challenges. Participants will provide feedback on uptake for new versions of C-CDA Templates: Considerations for errata releases, Value Set updates and possible C-CDA R 2. 2 SUNDAY, Q 3 © 2017 Health Level Seven ® International. All Rights Reserved. HL 7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U. S. TM Office. 36

Emergent and Parking Lot Topics Participants will conduct additional discussions for emergent or parking lot items. Participants interested in exploring other FHIR tracks may join with other track programs. SUNDAY, Q 4 © 2017 Health Level Seven ® International. All Rights Reserved. HL 7 and Health Level Seven are registered trademarks of Health Level Seven International. Reg. U. S. TM Office. 37
 Cms fhir connectathon
Cms fhir connectathon Day 1 day 2 day 3 day 4
Day 1 day 2 day 3 day 4 Hl7 connectathon 2019
Hl7 connectathon 2019 Ihe swf
Ihe swf Day 1 day 2 day 817
Day 1 day 2 day 817 My favourite day is monday
My favourite day is monday Concept map fhir
Concept map fhir Fhir activitydefinition
Fhir activitydefinition Devices on fhir
Devices on fhir Fhir browser
Fhir browser Fhir facade
Fhir facade Fhir data model
Fhir data model Fhir adalah
Fhir adalah Omop on fhir
Omop on fhir Davinci fhir implementation guide
Davinci fhir implementation guide Fhir allergyintolerance
Fhir allergyintolerance Oracle bpm
Oracle bpm Fhir smoking status
Fhir smoking status Fhir bpms engine
Fhir bpms engine Omop on fhir
Omop on fhir Fhir imaging study
Fhir imaging study Vonk fhir
Vonk fhir Cpg on fhir
Cpg on fhir Fhir version history
Fhir version history Trifolia on fhir
Trifolia on fhir Fhir provenance example
Fhir provenance example Fhir uscore
Fhir uscore Fhir mapping engine
Fhir mapping engine Immunization fhir
Immunization fhir L
L Role of transpiration
Role of transpiration Day to day maintenance
Day to day maintenance Tactique futsal
Tactique futsal Growing day by day
Growing day by day I live for jesus day after day
I live for jesus day after day Family sis schoolmax
Family sis schoolmax As your room gets messier day by day, entropy is
As your room gets messier day by day, entropy is Define seed dormancy
Define seed dormancy