HIVAIDS Prof OP rajoura HIV vs AIDS HIV





















- Slides: 46

HIV-AIDS Prof. OP rajoura

HIV v/s AIDS HIV: Human Immuno-deficiency Virus AIDS: Acquired Immuno Deficiency Syndrome A= not inherited I= immune system D= deficiency- inability to protect against illness S= syndrome, a group of symptoms or illnesses that occur as a result of HIV infection 18 -09 -2021 2

GLOBAL HISTORY • 1981 – beginning of the epidemic in United States - Centers for Disease Control and Prevention (CDC) published a report about five previously healthy homosexual men becoming infected with Pneumocystis pneumonia - Because the disease appeared to affect mostly homosexual men, officials initially called it Gay-related immune deficiency, or GRID. - CDC noted that this type of pneumonia had never affected people with uncompromised immune systems. • 1983 - Lymphadenopathy-Associated Virus (or LAV) retrovirus was discovered • 1984 - National Cancer Institute found the cause of AIDS to be retrovirus HTLV-III. 3

• 1985 - Food and Drug Administration licensed the first commercial blood test for HIV • 1986 - the International Committee on the Taxonomy of Viruses officially named the virus as HIV (human immunodeficiency virus) • 1988 - The World Health Organization declared December 1 st to be World AIDS Day. • 1991 - The red ribbon became an international symbol of AIDS awareness. • 2008 - Luc Antoine Montagnier was awarded Nobel Prize in Physiology and medicine for his discovery of the human immunodeficiency virus (HIV). 4

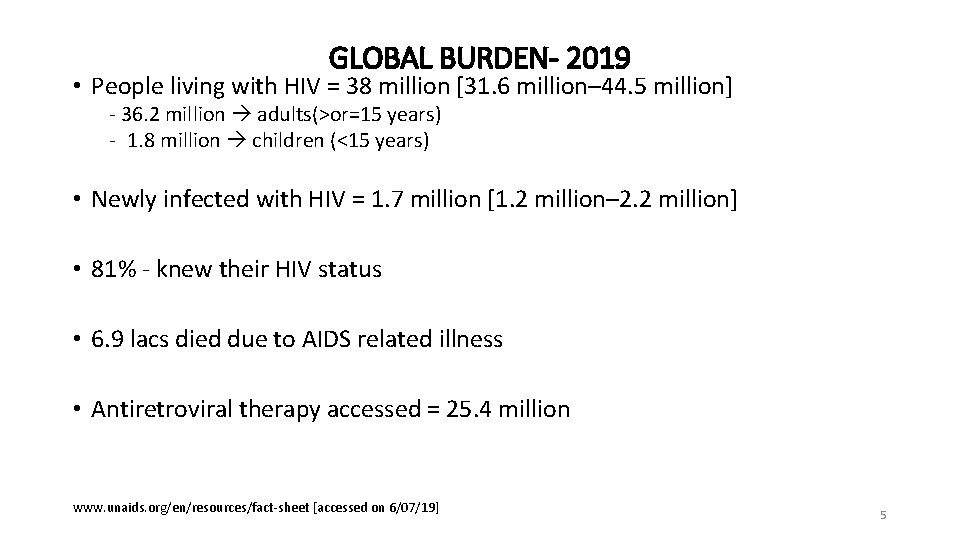
GLOBAL BURDEN- 2019 • People living with HIV = 38 million [31. 6 million– 44. 5 million] - 36. 2 million adults(>or=15 years) - 1. 8 million children (<15 years) • Newly infected with HIV = 1. 7 million [1. 2 million– 2. 2 million] • 81% - knew their HIV status • 6. 9 lacs died due to AIDS related illness • Antiretroviral therapy accessed = 25. 4 million www. unaids. org/en/resources/fact-sheet [accessed on 6/07/19] 5

INDIA • Suniti Solomon-first AIDS case was diagnosed in Chennai in 1986. • In 2009, she was awarded , “National Women Bio-scientist Award” by Ministry of Science and Technology, Govt. of India. • On 25 th January 2017, Govt. of India announced “Padma shri” award for her contribution towards Medicine • “what has been killing people with AIDS more is the stigma and discrimination” 18 -09 -2021 6

7

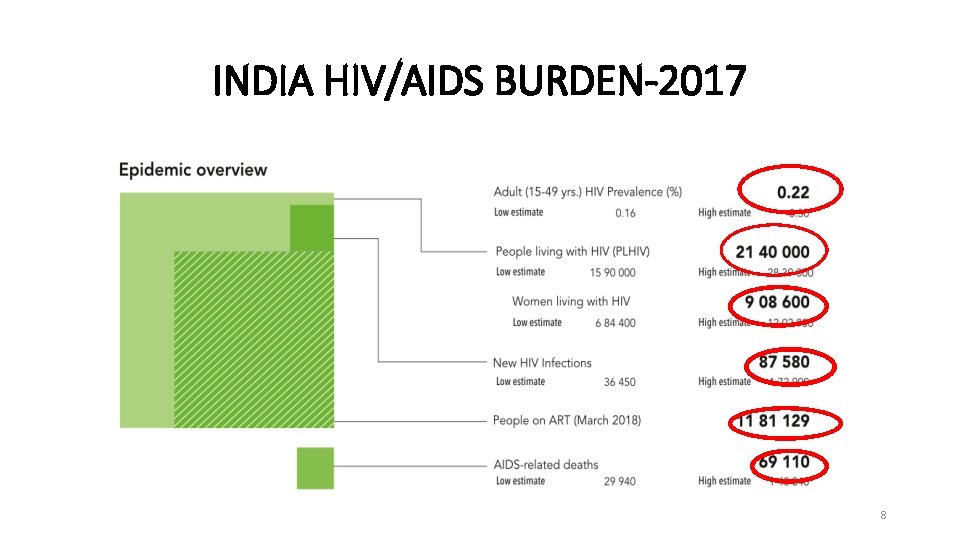
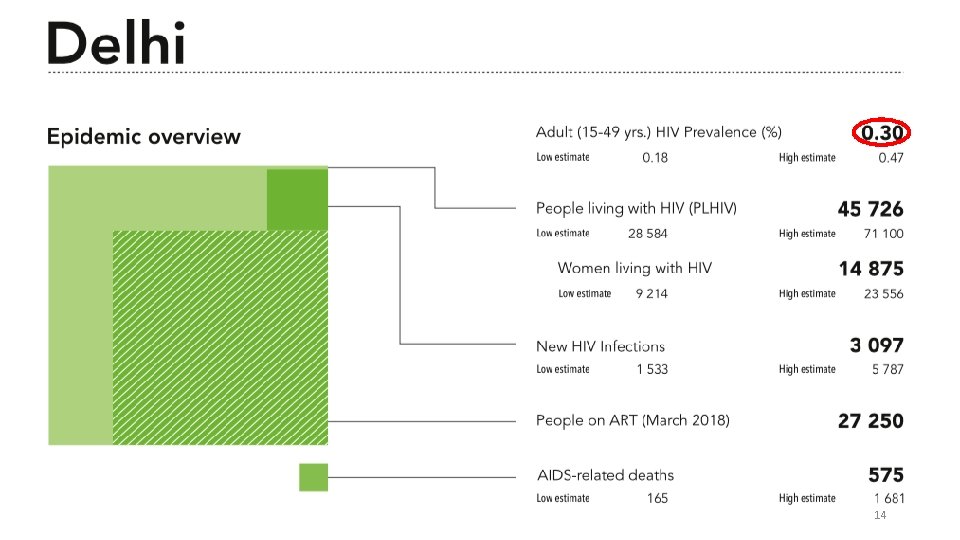
INDIA HIV/AIDS BURDEN-2017 8

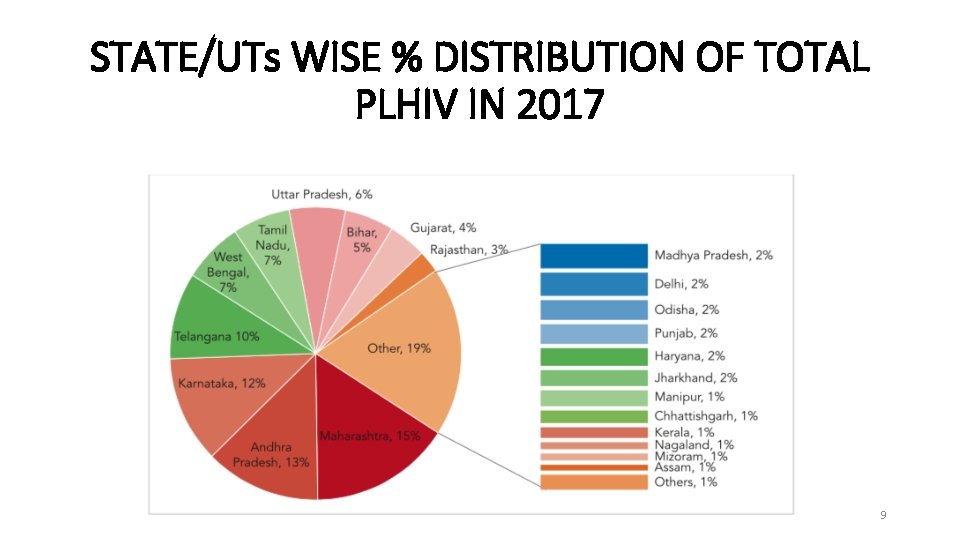
STATE/UTs WISE % DISTRIBUTION OF TOTAL PLHIV IN 2017 9

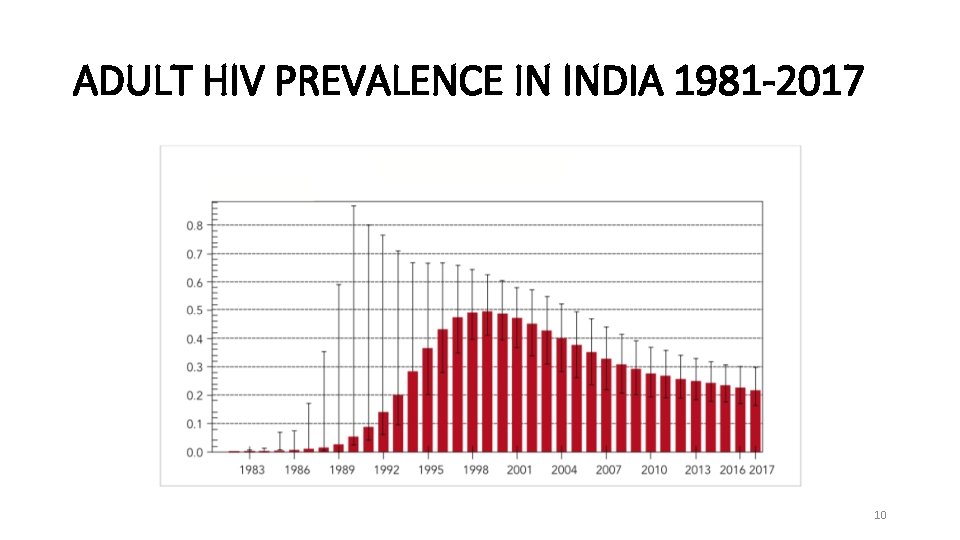
ADULT HIV PREVALENCE IN INDIA 1981 -2017 10

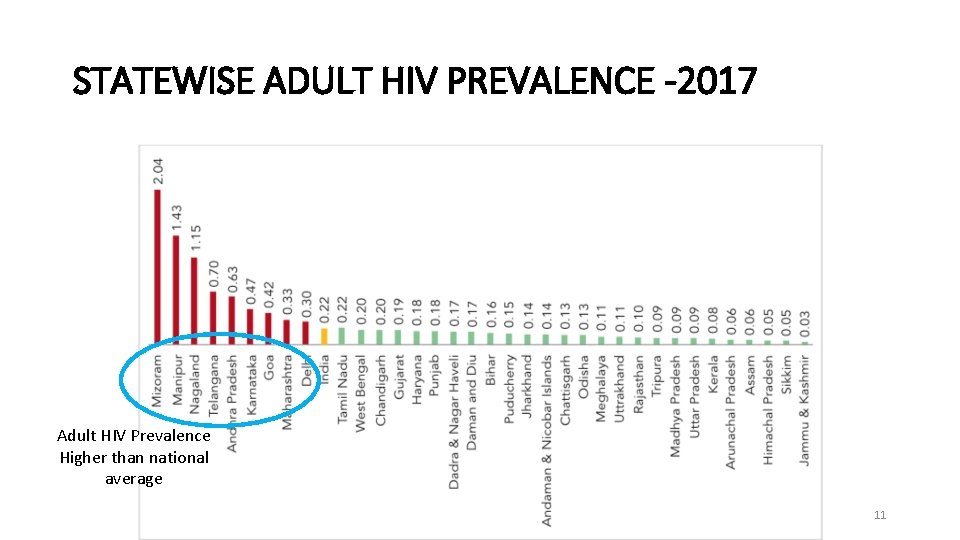
STATEWISE ADULT HIV PREVALENCE -2017 Adult HIV Prevalence Higher than national average 11

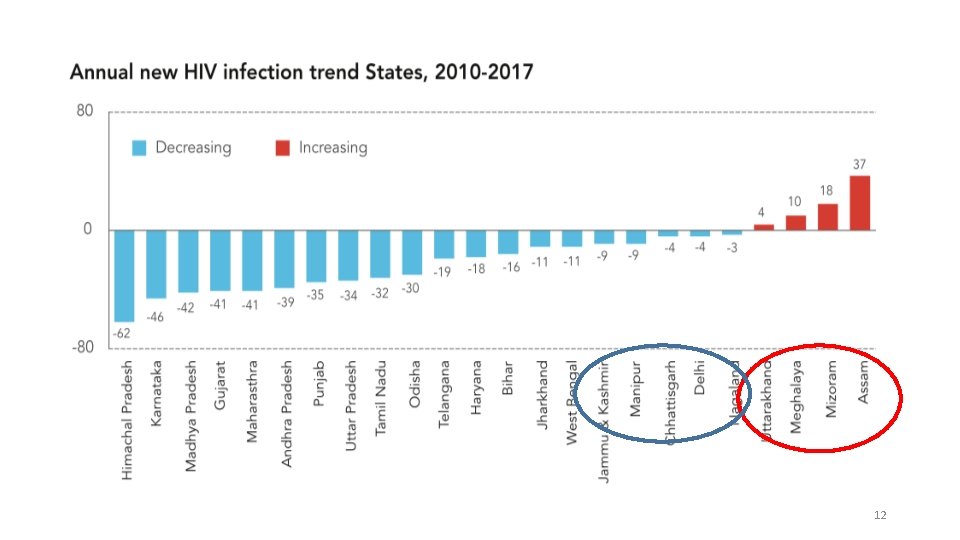
12

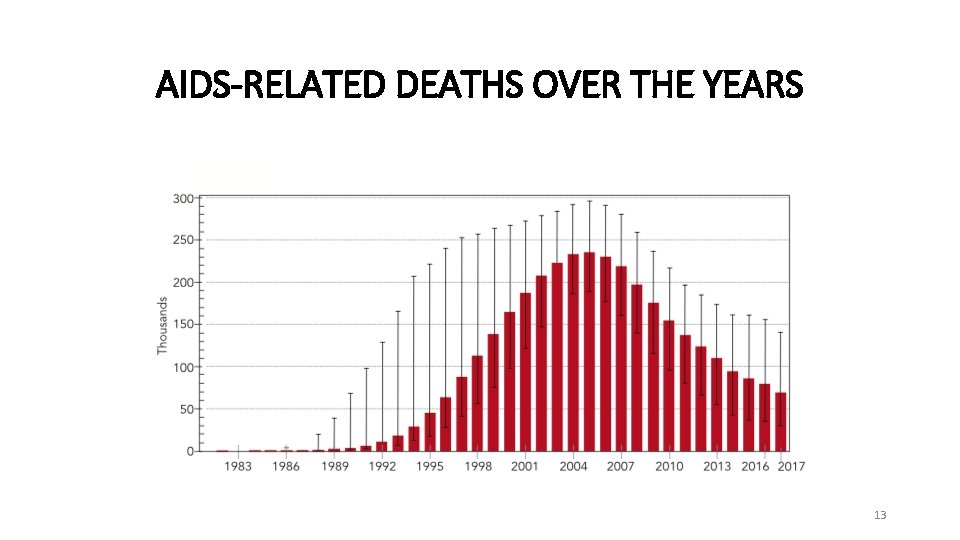
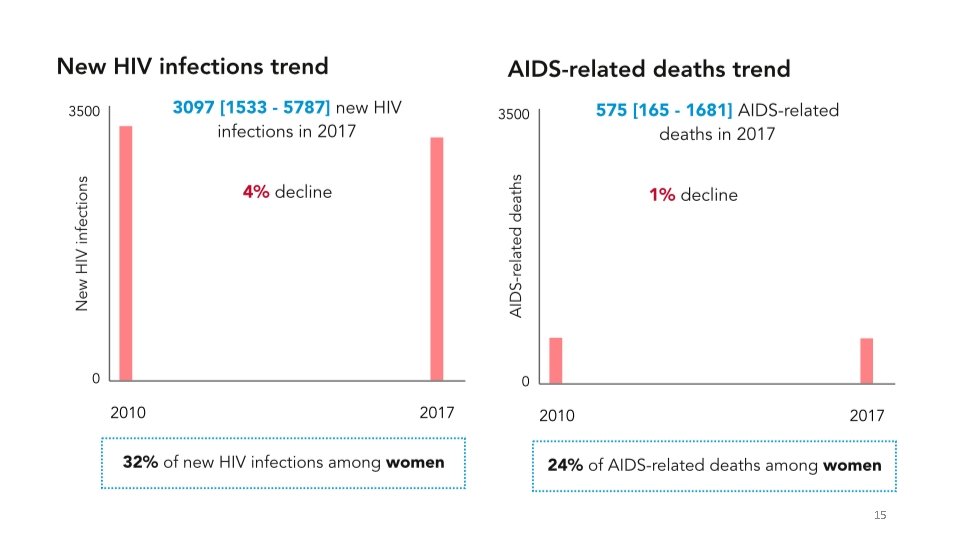
AIDS-RELATED DEATHS OVER THE YEARS 13

14

15

KEY TERMS • Key populations: are defined groups who, due to specific higher-risk behaviours, are at increased risk of HIV irrespective of the epidemic type or local context. 1) men who have sex with men 2) people who inject drugs 3) people in prisons and other closed settings 4) sex workers 5) transgender people. • Vulnerable populations are groups of people who are particularly vulnerable to HIV infection in certain situations or contexts, such as adolescents, orphans, street children, people with disabilities and migrant and mobile workers. • Men who have sex with men refers to all men who engage in sexual relations with other men. 17

• People who inject drugs: people who inject psychotropic (or psychoactive) substances for non-medical purposes. • Opioids, amphetamine-type stimulants, cocaine, hypno-sedatives and hallucinogens. • Injection may be through intravenous, intramuscular, subcutaneous or other injectable routes. • Transgender is an umbrella term for people whose gender identity and expression does not conform to the norms and expectations traditionally associated with the sex assigned to them at birth. • Includes people who are trans-sexual, transgender or otherwise gender nonconforming • Sex workers include female, male and transgender adults (18 years of age and above) who receive money or goods in exchange for sexual services, either regularly or occasionally. NOTE: People who self-inject medicines for medical purposes – referred to as “therapeutic injection” – are not included in this definition. The definition also does not include individuals who self-inject non-psychotropic substances, such as steroids or other hormones, for body shaping or improving athletic performance. 18

ETIOLOGY 18 -09 -2021 19

HIV • Enveloped ss. RNA Virus, • Genus: Lentivirus, Family Retroviridae, • Lentiviruses (HIV 1 and 2) • Attacks CD 4+ cells • Replicates in actively dividing T 4 lymphocytes. • The virus can remain in lymphoid tissues in latent phase until it is activated. • Unique ability to destroy T 4 Helper cells • Once a person gets infected, HIV remains in his body lifelong. • The person is a symptomless carrier for years before the symptoms actually appear. 18 -09 -2021 20

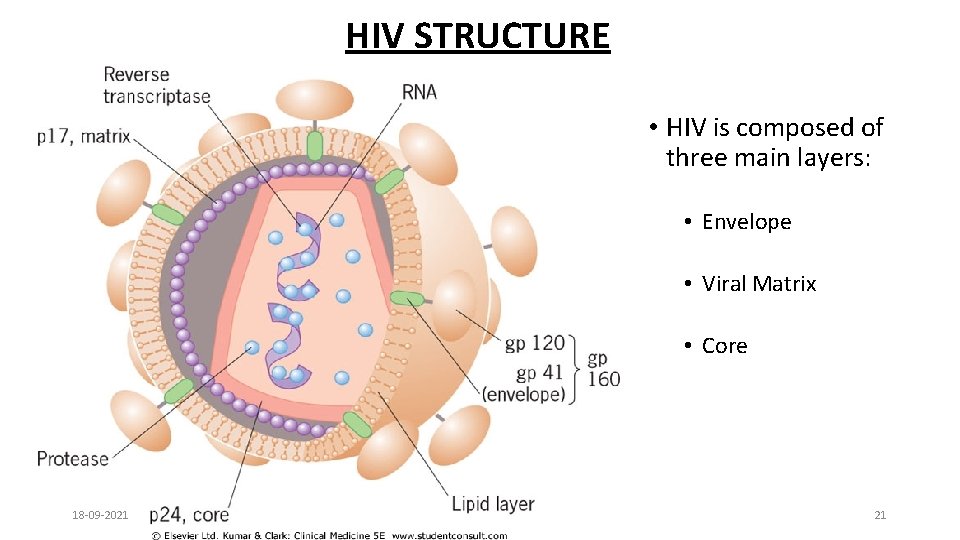
HIV STRUCTURE • HIV is composed of three main layers: • Envelope • Viral Matrix • Core 18 -09 -2021 21

HIV-1 AND HIV-2 • HIV-1 and HIV-2 • Transmitted though the same routes • Associated with similar opportunistic infections • HIV-1 is more common worldwide • Groups M, N, and O • Pandemic dominated by Group M § Group M comprised of subtypes A – J • HIV-2 is found primarily in West Africa, Mozambique and Angola • less easily transmitted • is develops more slowly • MTCT is relatively rare with HIV-2 18 -09 -2021 22

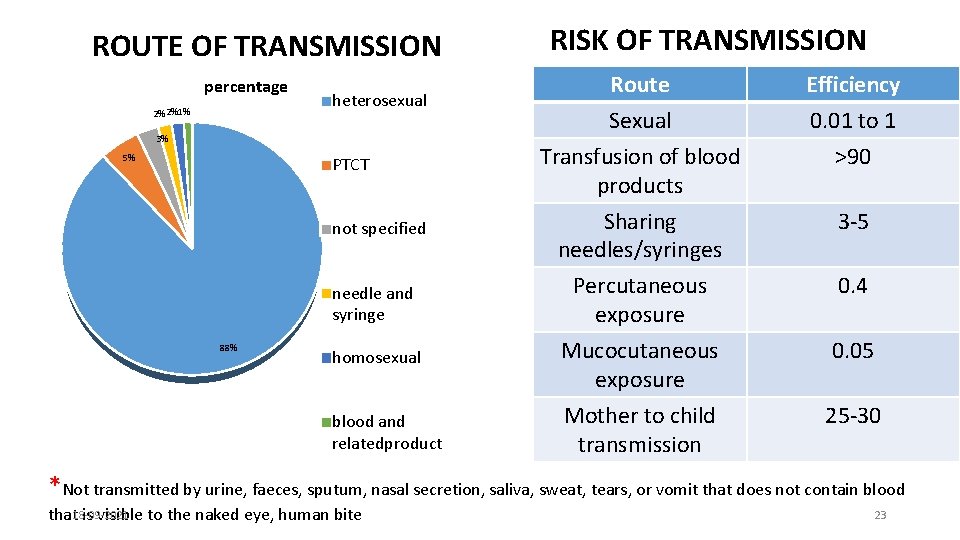
ROUTE OF TRANSMISSION percentage 2% 2%1% heterosexual 3% 5% PTCT not specified needle and syringe 88% homosexual blood and relatedproduct RISK OF TRANSMISSION Route Sexual Transfusion of blood products Efficiency 0. 01 to 1 >90 Sharing needles/syringes 3 -5 Percutaneous exposure Mucocutaneous exposure Mother to child transmission 0. 4 0. 05 25 -30 *Not transmitted by urine, faeces, sputum, nasal secretion, saliva, sweat, tears, or vomit that does not contain blood that 18 -09 -2021 is visible to the naked eye, human bite 23

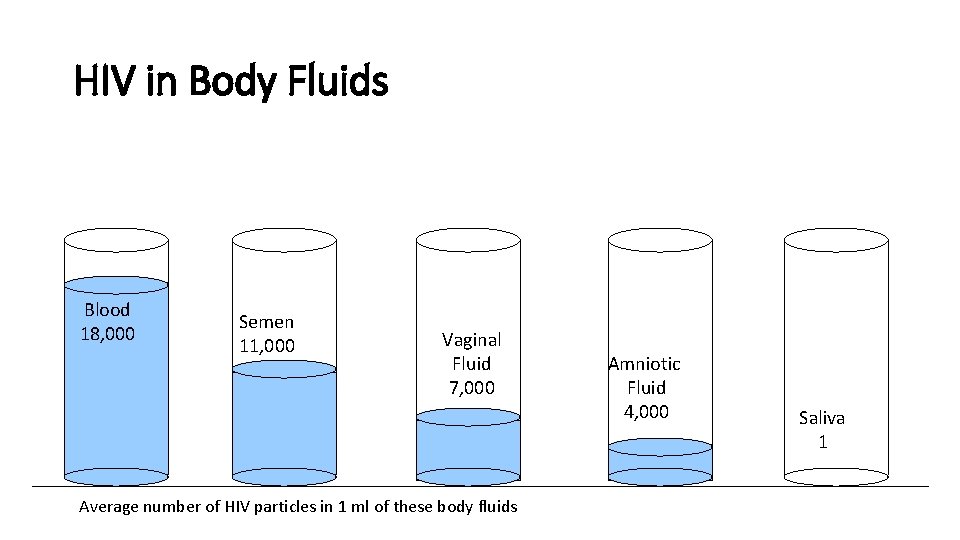
HIV in Body Fluids Blood 18, 000 Semen 11, 000 Vaginal Fluid 7, 000 Average number of HIV particles in 1 ml of these body fluids Amniotic Fluid 4, 000 Saliva 1

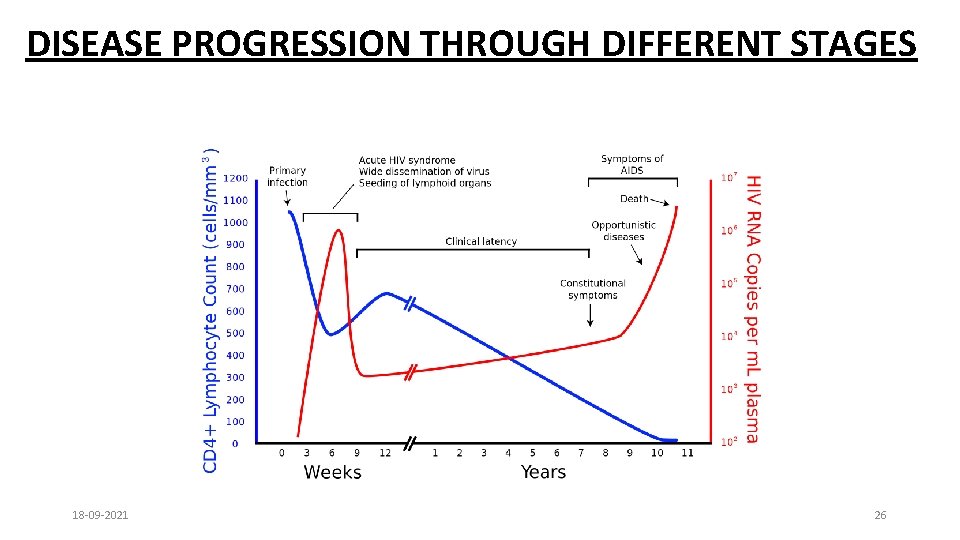
DISEASE PROGRESSION THROUGH DIFFERENT STAGES 18 -09 -2021 26

• Incubation period - Few months to 10 years or even more • 75% of people infected with HIV develop AIDS at the end of 10 years • Severity of illness determined by: • amount of virus in the blood (viral load) and • the degree of immune suppression (decreasing CD 4 count) • Higher the viral load, sooner immune suppression occurs 18 -09 -2021 27

• Window period: • Time between potential exposure to HIV infection and appearance of Antibodies in blood: 4 -12 weeks • Sero-conversion: Development of evidence of antibody response to a disease • Viral Load: The amount of HIV in the blood. 18 -09 -2021 28

CLINICAL FEATURES 18 -09 -2021 29

RISK FACTORS FOR TRANSMISSION OF HIV 1. Unsafe sex. 2. MSM (Men having Sex with Men). 3. IDU (Injection Drug User). 4. Migration & Mobility. 5. Low status of women. 6. Widespread stigma. 18 -09 -2021 30

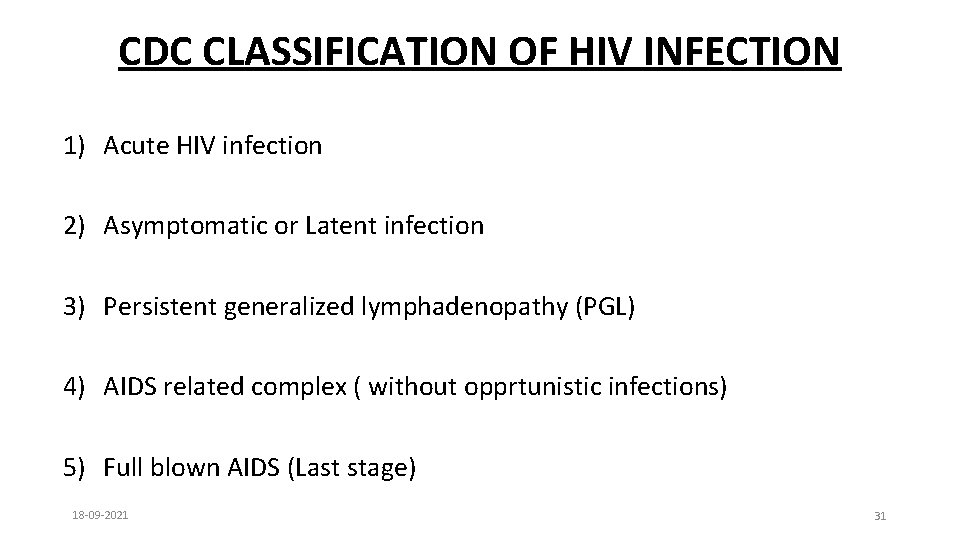
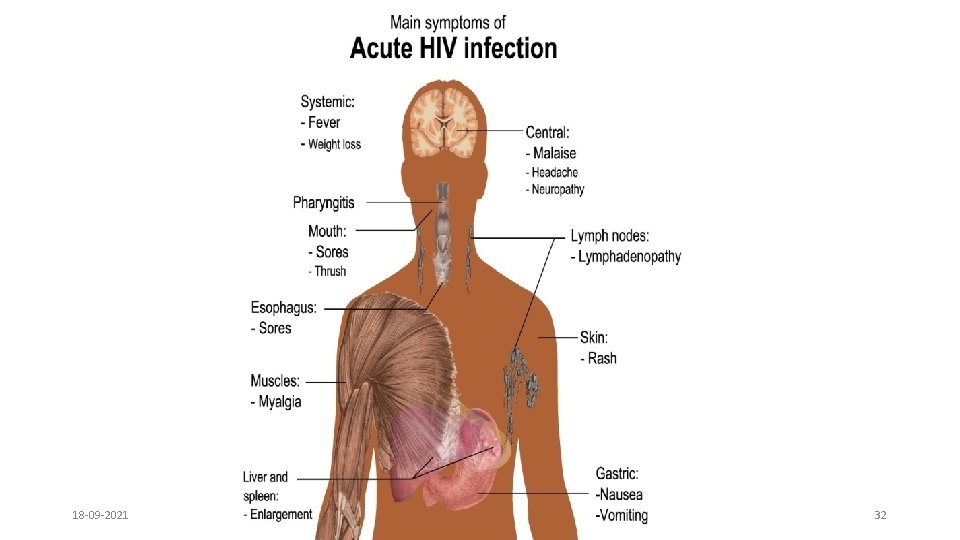
CDC CLASSIFICATION OF HIV INFECTION 1) Acute HIV infection 2) Asymptomatic or Latent infection 3) Persistent generalized lymphadenopathy (PGL) 4) AIDS related complex ( without opprtunistic infections) 5) Full blown AIDS (Last stage) 18 -09 -2021 31

18 -09 -2021 32

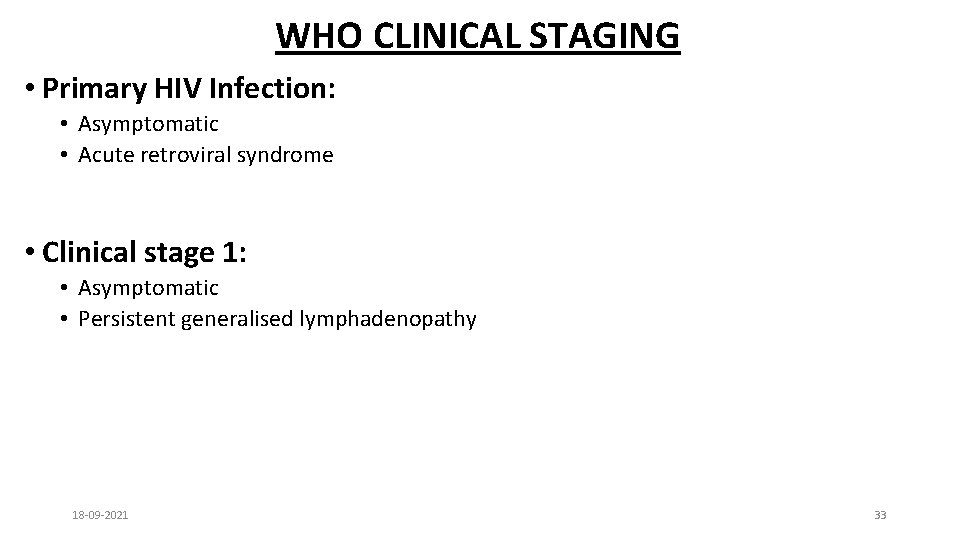
WHO CLINICAL STAGING • Primary HIV Infection: • Asymptomatic • Acute retroviral syndrome • Clinical stage 1: • Asymptomatic • Persistent generalised lymphadenopathy 18 -09 -2021 33

Clinical stage 2: • Moderate unexplained weight loss (<10% of presumed or measured body weight) • Recurrent respiratory tract infections (sinusitis, bronchitis, otitis media, pharyngitis) • Herpes zoster • Angular cheilitis • Recurrent oral ulcerations • Papular pruritic eruptions • Seborrhoeic dermatitis • Fungal nail infections of fingers 18 -09 -2021 34

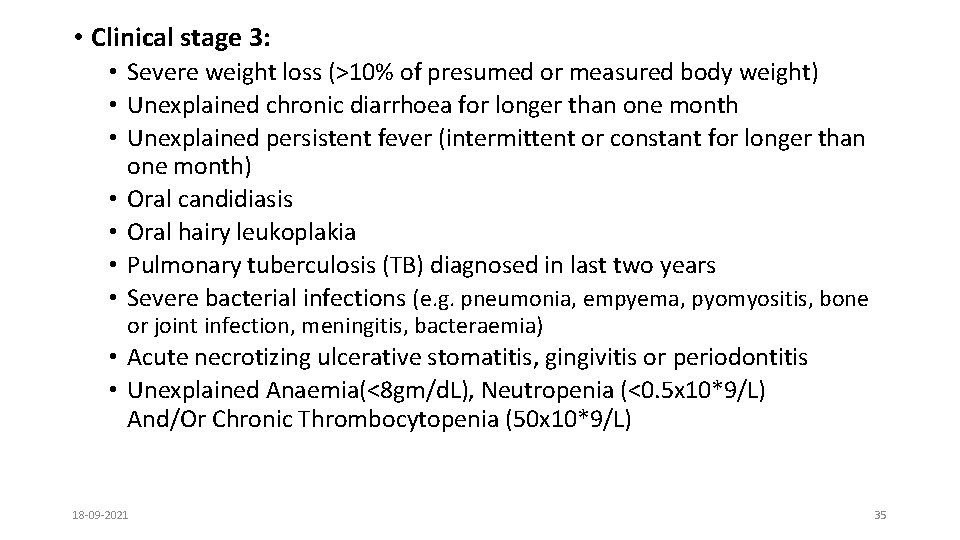
• Clinical stage 3: • Severe weight loss (>10% of presumed or measured body weight) • Unexplained chronic diarrhoea for longer than one month • Unexplained persistent fever (intermittent or constant for longer than one month) • Oral candidiasis • Oral hairy leukoplakia • Pulmonary tuberculosis (TB) diagnosed in last two years • Severe bacterial infections (e. g. pneumonia, empyema, pyomyositis, bone or joint infection, meningitis, bacteraemia) • Acute necrotizing ulcerative stomatitis, gingivitis or periodontitis • Unexplained Anaemia(<8 gm/d. L), Neutropenia (<0. 5 x 10*9/L) And/Or Chronic Thrombocytopenia (50 x 10*9/L) 18 -09 -2021 35

• Clinical stage 4: • HIV wasting syndrome • Pneumocystis carinii pneumonia (PCP) • Recurrent severe bacterial pneumonia • Chronic Herpes Simplex infection ( Orolabial, Genital or ano-rectal of <1 months duration or visceral at any site) • Candidiasis (Esophageal/ Trachea/ Bronchi/Lungs) • EPTB • Kaposis Sarcoma • CMV infection (Retina or other organs) • CNS Toxoplasmosis • HIV Encephalopathy 18 -09 -2021 36

• Extra-Pulmonary Cryptoccocosis • Disseminated Mycosis • Recurrent Septicaemia • Lymphoma (B-cell Non Hodgkins) • Invasive Cervical Carcinoma • Atypical disseminated Leishmaniasis • Symptomatic HIV-associated Nephropathy or Cardiomyopathy. 18 -09 -2021 37

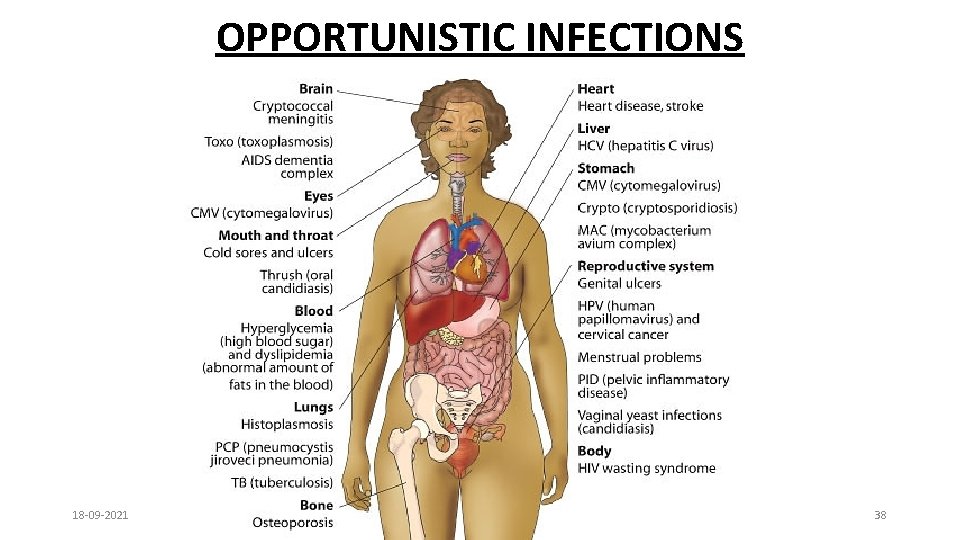
OPPORTUNISTIC INFECTIONS 18 -09 -2021 38

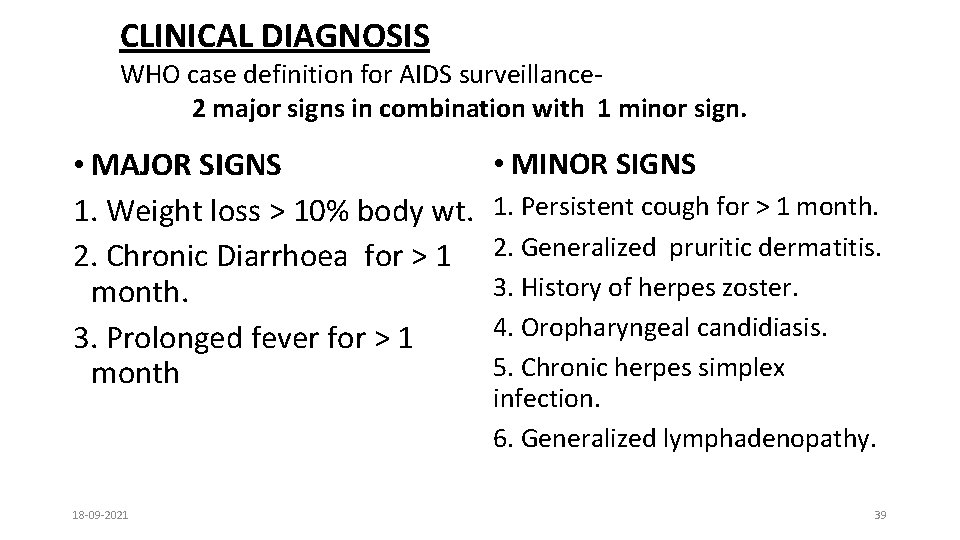
CLINICAL DIAGNOSIS WHO case definition for AIDS surveillance 2 major signs in combination with 1 minor sign. • MAJOR SIGNS 1. Weight loss > 10% body wt. 2. Chronic Diarrhoea for > 1 month. 3. Prolonged fever for > 1 month 18 -09 -2021 • MINOR SIGNS 1. Persistent cough for > 1 month. 2. Generalized pruritic dermatitis. 3. History of herpes zoster. 4. Oropharyngeal candidiasis. 5. Chronic herpes simplex infection. 6. Generalized lymphadenopathy. 39

LABORATORY DIAGNOSIS • Viral infection • Viral Load • p 24 Antigen • Immune response • Antibody (Ig. G, Ig. M) • Cellular response (CD 4) 18 -09 -2021 41

TREATMENT 18 -09 -2021 42

GOALS OF ART • Clinical goals : Prolongation of life and improvement in quality of life • Virological goals : Greatest possible reduction in viral load for as long as possible • Immunological goals : Immune reconstitution that is both quantitative and qualitative • Therapeutic goals : Rational sequencing of drugs in to achieve: • clinical, virological and immunological goals while maintaining treatment options, limiting drug toxicity and facilitating adherence • Reduction of HIV transmission in individuals : by suppression of viral load 18 -09 -2021

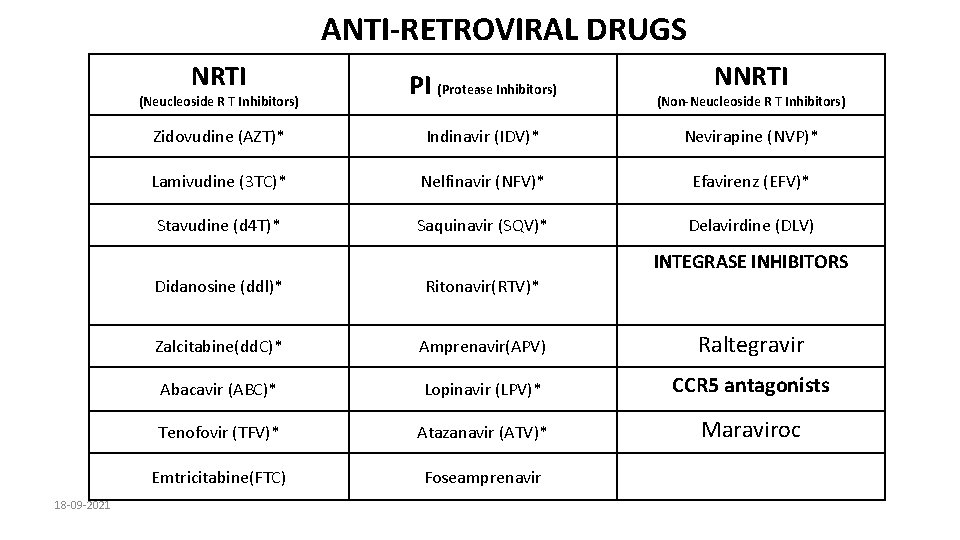
ANTI-RETROVIRAL DRUGS NRTI PI (Protease Inhibitors) Zidovudine (AZT)* Indinavir (IDV)* Nevirapine (NVP)* Lamivudine (3 TC)* Nelfinavir (NFV)* Efavirenz (EFV)* Stavudine (d 4 T)* Saquinavir (SQV)* Delavirdine (DLV) (Neucleoside R T Inhibitors) NNRTI (Non-Neucleoside R T Inhibitors) INTEGRASE INHIBITORS 18 -09 -2021 Didanosine (ddl)* Ritonavir(RTV)* Zalcitabine(dd. C)* Amprenavir(APV) Raltegravir Abacavir (ABC)* Lopinavir (LPV)* CCR 5 antagonists Tenofovir (TFV)* Atazanavir (ATV)* Maraviroc Emtricitabine(FTC) Foseamprenavir

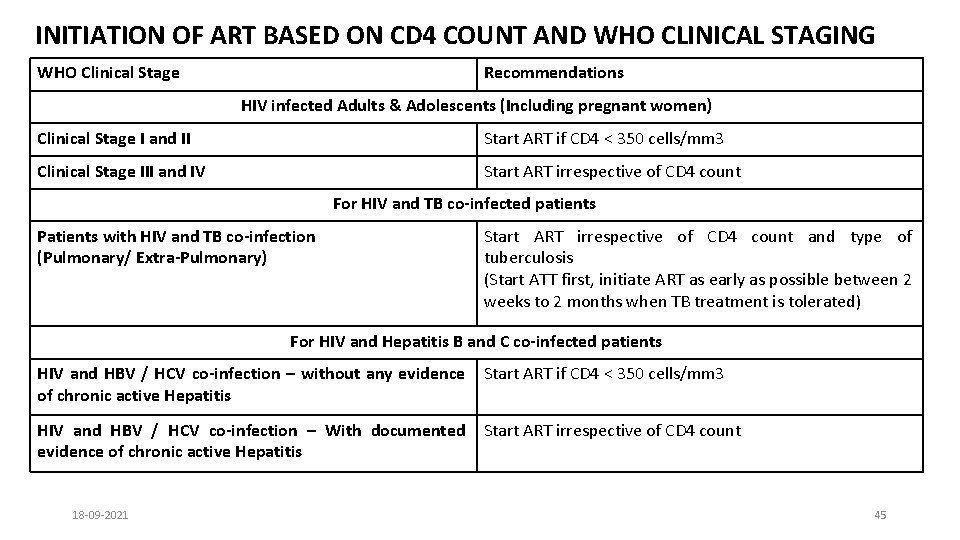
INITIATION OF ART BASED ON CD 4 COUNT AND WHO CLINICAL STAGING WHO Clinical Stage Recommendations HIV infected Adults & Adolescents (Including pregnant women) Clinical Stage I and II Start ART if CD 4 < 350 cells/mm 3 Clinical Stage III and IV Start ART irrespective of CD 4 count For HIV and TB co-infected patients Patients with HIV and TB co-infection (Pulmonary/ Extra-Pulmonary) Start ART irrespective of CD 4 count and type of tuberculosis (Start ATT first, initiate ART as early as possible between 2 weeks to 2 months when TB treatment is tolerated) For HIV and Hepatitis B and C co-infected patients HIV and HBV / HCV co-infection – without any evidence of chronic active Hepatitis Start ART if CD 4 < 350 cells/mm 3 HIV and HBV / HCV co-infection – With documented evidence of chronic active Hepatitis Start ART irrespective of CD 4 count 18 -09 -2021 45

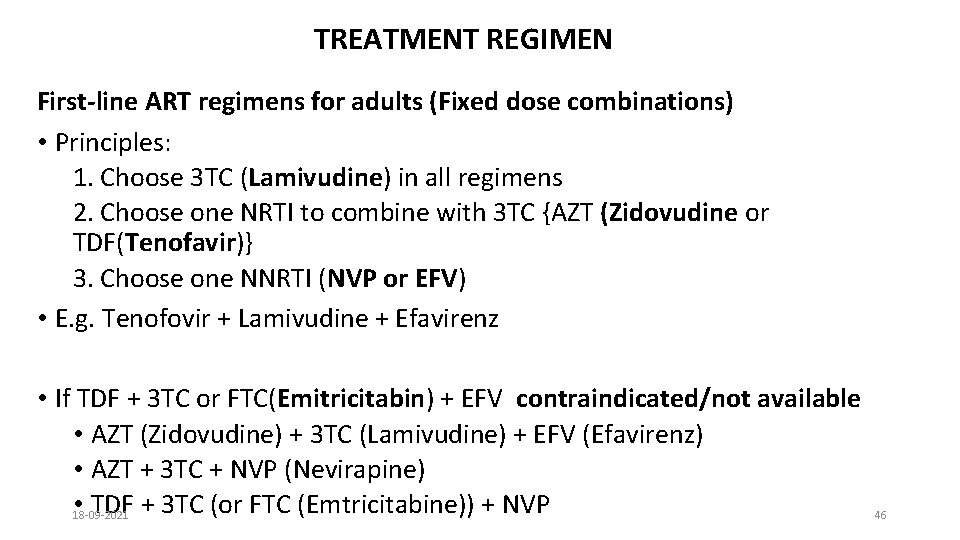
TREATMENT REGIMEN First-line ART regimens for adults (Fixed dose combinations) • Principles: 1. Choose 3 TC (Lamivudine) in all regimens 2. Choose one NRTI to combine with 3 TC {AZT (Zidovudine or TDF(Tenofavir)} 3. Choose one NNRTI (NVP or EFV) • E. g. Tenofovir + Lamivudine + Efavirenz • If TDF + 3 TC or FTC(Emitricitabin) + EFV contraindicated/not available • AZT (Zidovudine) + 3 TC (Lamivudine) + EFV (Efavirenz) • AZT + 3 TC + NVP (Nevirapine) • 18 -09 -2021 TDF + 3 TC (or FTC (Emtricitabine)) + NVP 46

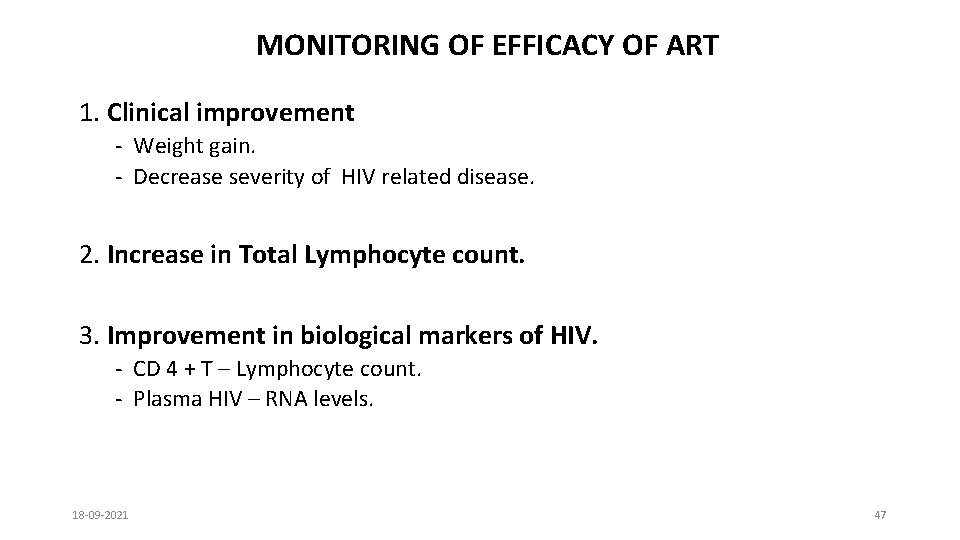
MONITORING OF EFFICACY OF ART 1. Clinical improvement - Weight gain. - Decrease severity of HIV related disease. 2. Increase in Total Lymphocyte count. 3. Improvement in biological markers of HIV. - CD 4 + T – Lymphocyte count. - Plasma HIV – RNA levels. 18 -09 -2021 47

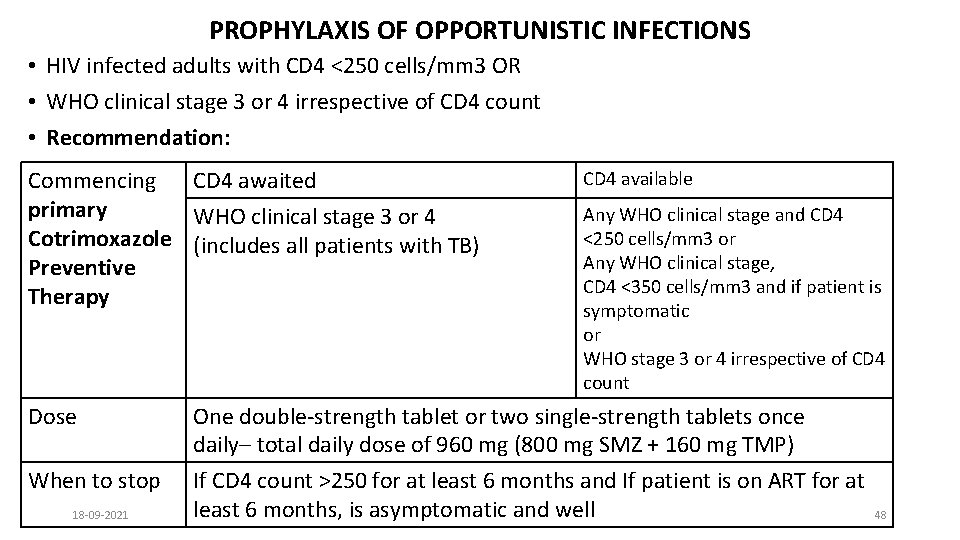
PROPHYLAXIS OF OPPORTUNISTIC INFECTIONS • HIV infected adults with CD 4 <250 cells/mm 3 OR • WHO clinical stage 3 or 4 irrespective of CD 4 count • Recommendation: Commencing CD 4 awaited primary WHO clinical stage 3 or 4 Cotrimoxazole (includes all patients with TB) Preventive Therapy CD 4 available Any WHO clinical stage and CD 4 <250 cells/mm 3 or Any WHO clinical stage, CD 4 <350 cells/mm 3 and if patient is symptomatic or WHO stage 3 or 4 irrespective of CD 4 count Dose One double-strength tablet or two single-strength tablets once daily– total daily dose of 960 mg (800 mg SMZ + 160 mg TMP) When to stop If CD 4 count >250 for at least 6 months and If patient is on ART for at least 6 months, is asymptomatic and well 18 -09 -2021 48

Thank you 18 -09 -2021 49
 Chapter 25 sexually transmitted infections and hiv/aids
Chapter 25 sexually transmitted infections and hiv/aids Kasus hiv aids
Kasus hiv aids Chapter 24 sexually transmitted diseases and hiv/aids
Chapter 24 sexually transmitted diseases and hiv/aids Causative organism of hiv/aids
Causative organism of hiv/aids Test wiedzy o hiv i aids
Test wiedzy o hiv i aids Chapter 17 oral pathology short answer questions
Chapter 17 oral pathology short answer questions Adventist aids international ministry
Adventist aids international ministry Choroby wskaznikowe aids
Choroby wskaznikowe aids Ergogenic aids definition
Ergogenic aids definition Runway turn pad marking
Runway turn pad marking Consumer aids
Consumer aids Transparencies visual aids
Transparencies visual aids Types of training aids
Types of training aids Arl para aids
Arl para aids National aids council tenders
National aids council tenders Aids
Aids Advanced diagnostic aids in periodontics
Advanced diagnostic aids in periodontics Organizational text features
Organizational text features Uganda aids rate
Uganda aids rate Poor visual aids
Poor visual aids Tips for augmentative and alternative communication success
Tips for augmentative and alternative communication success Teaching aids examples
Teaching aids examples Aids africa essay
Aids africa essay Teaching aids
Teaching aids Ergogenic aids examples
Ergogenic aids examples Auxiliary aid
Auxiliary aid Extrinsic aids examples
Extrinsic aids examples Kostuumschoenen kopen
Kostuumschoenen kopen Hearing aids ennis
Hearing aids ennis Tahap arc (aids related complex)
Tahap arc (aids related complex) Sumptoms of hiv
Sumptoms of hiv Av aids meaning
Av aids meaning Importance of teaching aids
Importance of teaching aids For military
For military Acquired immunity
Acquired immunity Internal aids of construction
Internal aids of construction Visual aids for denoting obstacles
Visual aids for denoting obstacles Nc fast job aids
Nc fast job aids Extrinsic and intrinsic aids to statutory interpretation
Extrinsic and intrinsic aids to statutory interpretation Visual aids in airport
Visual aids in airport Graphic aids definition
Graphic aids definition Aids alliance raleigh nc
Aids alliance raleigh nc Hearing aids picture
Hearing aids picture Use of epidiascope in education
Use of epidiascope in education Aids arms inc
Aids arms inc Powell v kempton park racecourse
Powell v kempton park racecourse Ergogenic aids definition
Ergogenic aids definition





































































