HIV TESTING PROTOCOLS D Vijay kumar Dist Sales























- Slides: 37

HIV TESTING PROTOCOLS D. Vijay kumar Dist. Sales Manager Abbott Hyderabad.

Informed consent prior to testing is essential to deal with issues like- §Confidentiality §Discrimination §Victimization §Psychological harm * HIV testing – voluntary Mandatory testing – counterproductive

Purpose of HIV testing

§ Transfusion safety § Epidemiological - Sentinel surveillance § Diagnostic purpose § Voluntary testing § Research

PPTCT Pregnant HIV infected women can§ make informed decisions about dealing with pregnancy § receive appropriate and timely interventions to decrease MTCT § ensure safe delivery § secure early access to HIV care and treatment § educated in prevention of HIV transmission § receive follow-up health care for self and child Pregnant HIV non infected women can be educated and counselled to remain uninfected

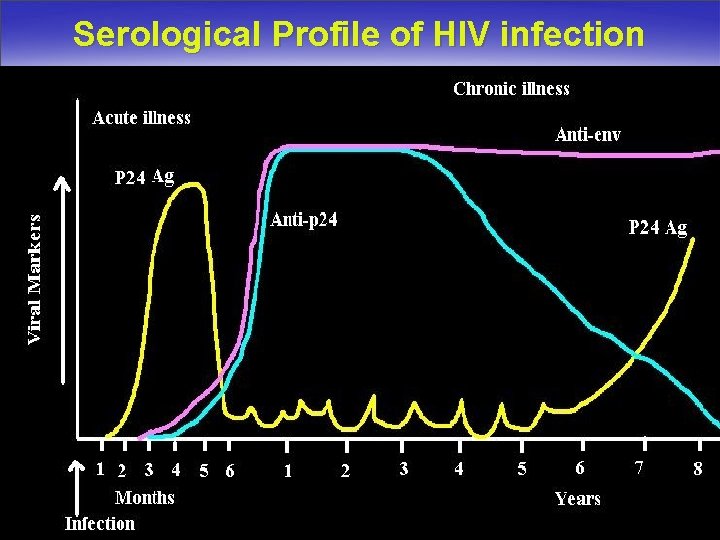
Serological Profile of HIV infection

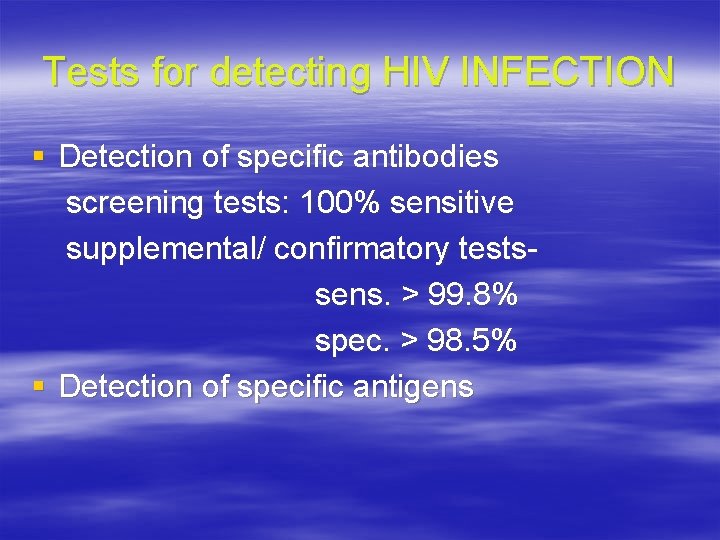
Tests for detecting HIV INFECTION § Detection of specific antibodies screening tests: 100% sensitive supplemental/ confirmatory testssens. > 99. 8% spec. > 98. 5% § Detection of specific antigens

SELECTION OF TESTS : BASED ON SENSITIVITY , SPECIFICITY, EFFICIENCY, PPV & NPV Sensitivity – Accuracy with which a test can confirm the presence of an infection. Test with high sensitivity – few false negatives TP Sensitivity = x 100 TP + FN

SPECIFICITYAccuracy with which the test can confirm the absence of an infection test with high specificity – few false positives used for diagnosing infection in an individual TN Specificity = X 100 TN + FP

Efficiency - ability of a test to correctly identify all positives as positives all negatives as negatives TP + TN Efficiency = X 100 TP + FN + TN + FP

Predictive values PPV – identifies ACTUALLY infected individuals TP X 100 TP + FP NPV – identifies ACTUAL non infected TN X 100 TN + FN

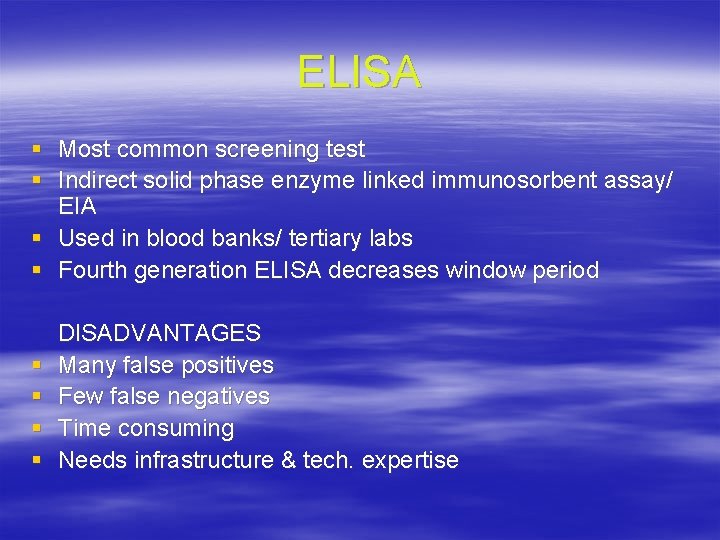
ELISA § Most common screening test § Indirect solid phase enzyme linked immunosorbent assay/ EIA § Used in blood banks/ tertiary labs § Fourth generation ELISA decreases window period § § DISADVANTAGES Many false positives Few false negatives Time consuming Needs infrastructure & tech. expertise

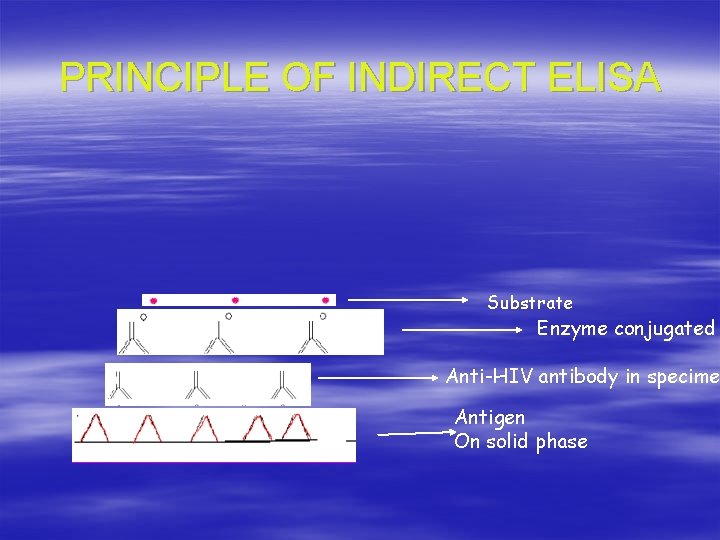
PRINCIPLE OF INDIRECT ELISA Substrate Enzyme conjugated Anti-HIV antibody in specime Antigen On solid phase

ELISA cont. § False positives: auto immune diseases multiple pregnancies hematologic malignancies primary biliary cirrhosis alcoholic hepatitis CRF § False negatives window period immunosuppressive therapy malignant disorders late stage disease technical errors

Rapid tests § Dot blot assay- immuno concentration method Retroquic -line assay Tridot - dot assay § Immunocomb assay – dipstick/comb ELISA based HIV comb coomb AIDS § Immunochromatography – lateral flow assays Determine, Unigold, Hemastrip § Particle aggluttination – Capillus, Serodia § ELISA based - EIA

Comb AIDS ØDot immunoassay for HIV 1&2 using whole blood, serum or plasma. Comb with 8 teeth- Megenta red spot ØSynthetic& recombinant peptides used ØTwo spots-- Control spot & test spot. NON-REACTIVE For HIV-1 &2 INVALID TEST

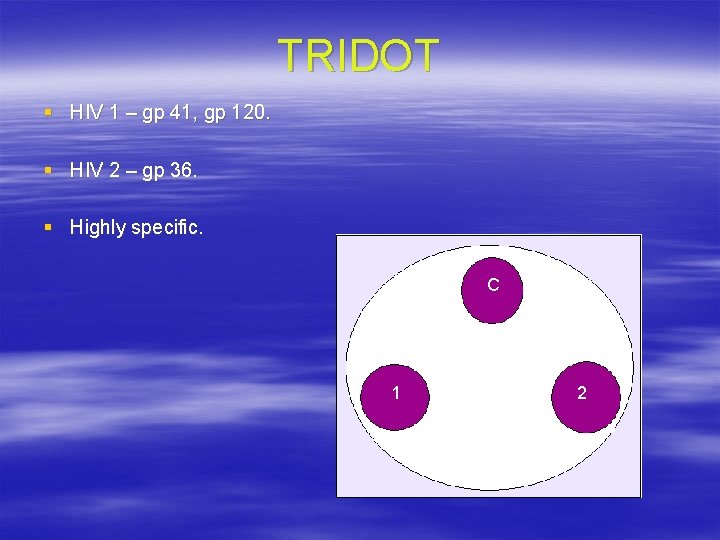
TRIDOT § HIV 1 – gp 41, gp 120. § HIV 2 – gp 36. § Highly specific. C 1 2

RETROQUIC HIV

HIV EIA Comb

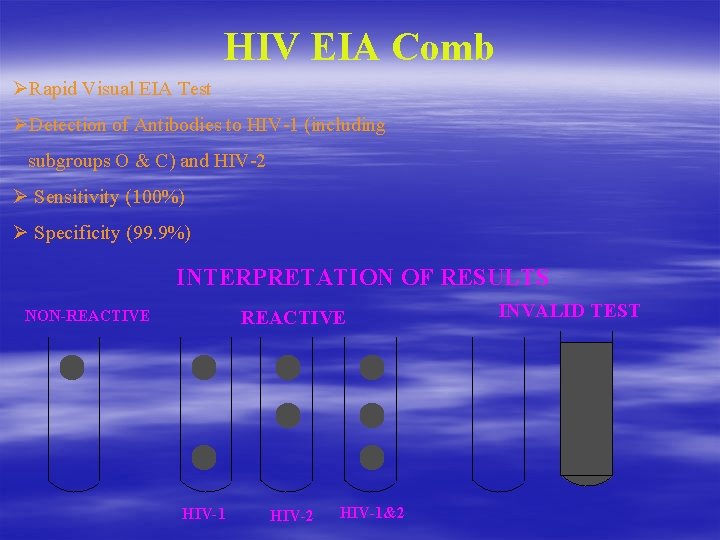
HIV EIA Comb ØRapid Visual EIA Test ØDetection of Antibodies to HIV-1 (including subgroups O & C) and HIV-2 Ø Sensitivity (100%) Ø Specificity (99. 9%) INTERPRETATION OF RESULTS NON-REACTIVE HIV-1 HIV-2 HIV-1&2 INVALID TEST

SUPPLEMENTAL TESTS § § 1. Rapid tests. 2. Western blot. 3. Immunoblot. 4. Line immunoassay. § WB/IB/LIA: highly specific but Expensive Labour intensive Needs expertise Equivocal/indeterminate results.

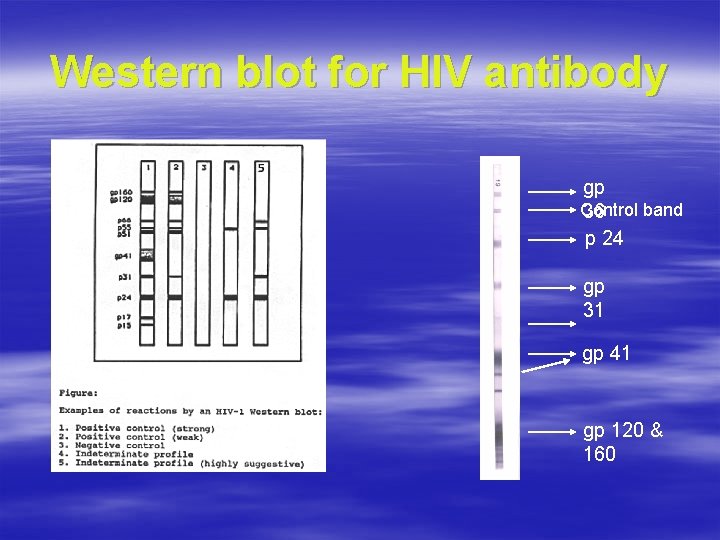
Western Blot for HIV § Delineates the antibody profile of reactive serum WB Rotator platform § Used to grade intensity of ab response – Qualitatively – Quantitatively § Procedure based on principle of ELISA WB strips

Western blot for HIV antibody gp Control band 36 p 24 gp 31 gp 41 gp 120 & 160

TESTS DONE IN VCCTC, PPTCT & BLOOD BANK VCCTC & PPTCT: Test – I: HIV Comb / Comb Aids – RS Test – II: Tridot / Retroquic Test – III: EIA Comb FOR SURVEILLANCE: Test – I: HIV Comb / Comb Aids – RS Test – II: Tridot / Retroquic BLOOD BANK: Only Test: Microlisa HIV

STRATEGIES/ALGORITHMS OF HIV TESTING 1. Screening ELISA/Rapid tests – used in strategy I, III. 2. Supplemental tests – E/R & Western Blot. Strategy I (for transfusion/transplantation safety) one test kit required AI A 1 + A 1 positive negative

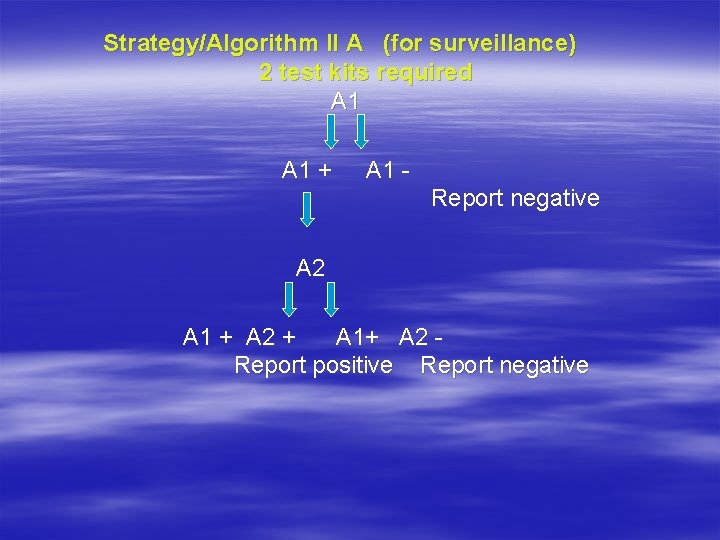
Strategy/Algorithm II A (for surveillance) 2 test kits required A 1 + A 1 Report negative A 2 A 1 + A 2 + A 1+ A 2 Report positive Report negative

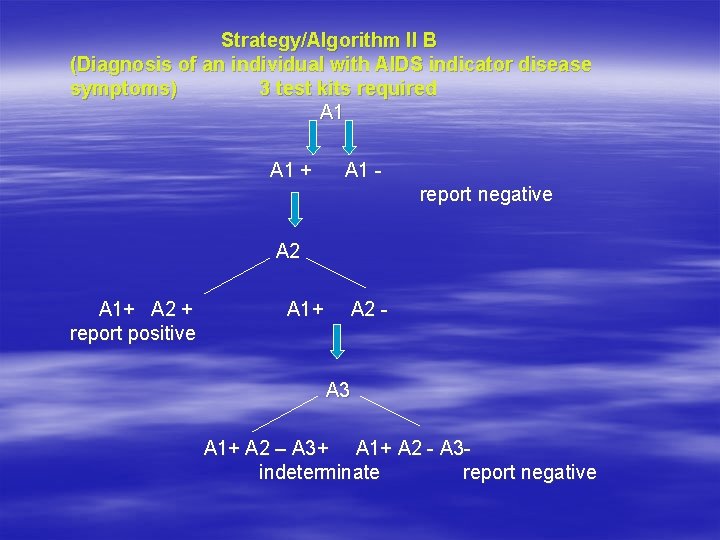
Strategy/Algorithm II B (Diagnosis of an individual with AIDS indicator disease symptoms) 3 test kits required A 1 + A 1 report negative A 2 A 1+ A 2 + report positive A 1+ A 2 - A 3 A 1+ A 2 – A 3+ A 1+ A 2 - A 3 indeterminate report negative

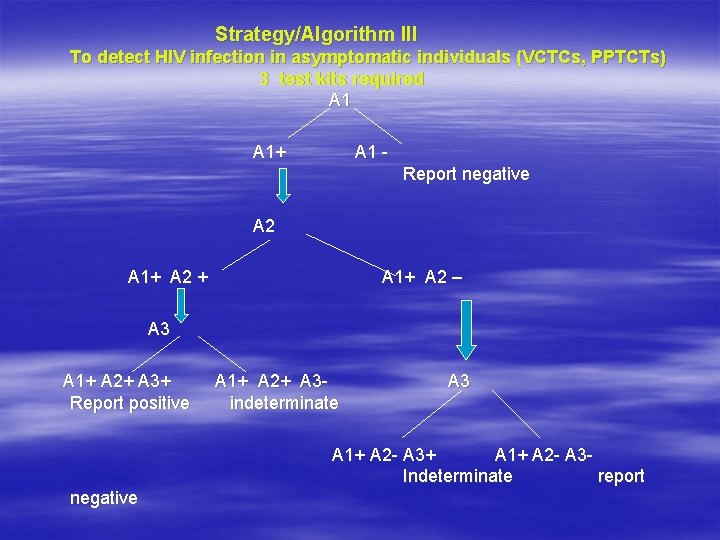
Strategy/Algorithm III To detect HIV infection in asymptomatic individuals (VCTCs, PPTCTs) 3 test kits required A 1+ A 1 Report negative A 2 A 1+ A 2 + A 1+ A 2 – A 3 A 1+ A 2+ A 3+ Report positive A 1+ A 2+ A 3 indeterminate A 3 A 1+ A 2 - A 3+ A 1+ A 2 - A 3 Indeterminate report negative

§ INDETERMINATE STATUS: Repeat test after 14 -28 days. Results continue to be indeterminate – WB/PCR refer to NRL. § EQUIVOCAL WB: Rpt. WB after 2 weeks 4 weeks 12 weeks one year. Correlate with high risk behaviour & clinical parameters.

TESTS TO DETECT ANTIGENS § P 24 Antigen: Uncomplexed in serum, plasma, CSF, cell culture. Indicates Active infection especially in newborn. Resolves equivocal WB. Window period. CNS disease. Immune collapse. Monitoring response to ART. Method: EIA Disadvantages: Expensive. Limited sensitivity. Failure to detect HIV 2. Failure to detect Ag when complexed with Antibody

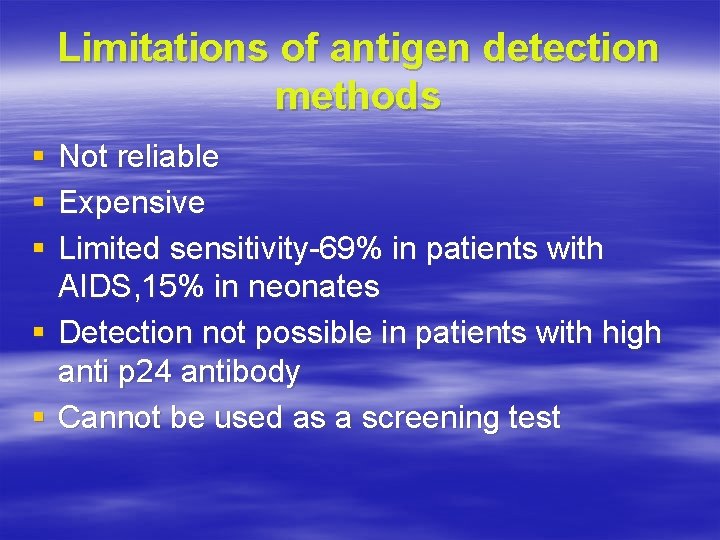
Limitations of antigen detection methods § § § Not reliable Expensive Limited sensitivity-69% in patients with AIDS, 15% in neonates Detection not possible in patients with high anti p 24 antibody Cannot be used as a screening test

PCR § Highly specific test-more than 95% § Highly sensitive-infants over 1 month § Detects proviral DNA. § Detects both latent viral infection and active viral transcriptipn. § Detects viral load. § Detects both HIV 1 & HIV 2.

PCR AS VIRAL ASSAYS IN INFANTS § Counselling for infant feeding & therapeutic intervention. § First done at 6 weeks. § Not Breast-fed, to say not infected: 2 negative test after 1 month (include 1 at 4 months) § Not done as part of PPTCT in India. § If symptoms occur at < 18 months: go for viral assays.

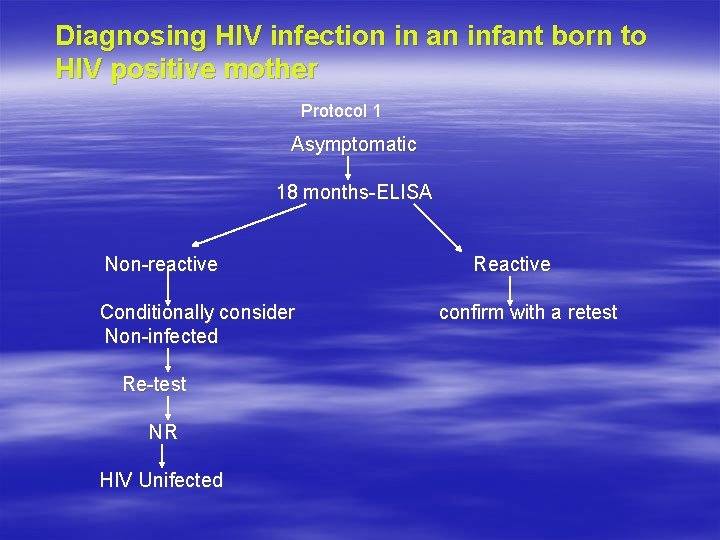
Diagnosing HIV infection in an infant born to HIV positive mother Protocol 1 Asymptomatic 18 months-ELISA Non-reactive Conditionally consider Non-infected Re-test NR HIV Unifected Reactive confirm with a retest

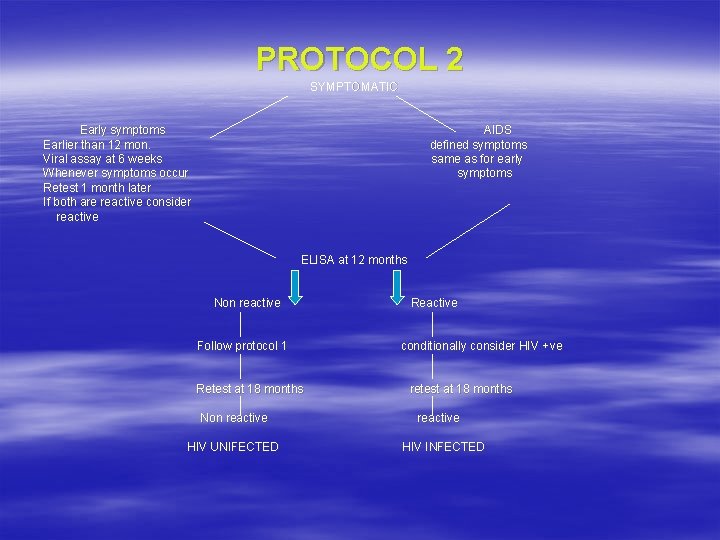
PROTOCOL 2 SYMPTOMATIC Early symptoms Earlier than 12 mon. Viral assay at 6 weeks Whenever symptoms occur Retest 1 month later If both are reactive consider reactive AIDS defined symptoms same as for early symptoms ELISA at 12 months Non reactive Follow protocol 1 Retest at 18 months Non reactive HIV UNIFECTED Reactive conditionally consider HIV + ve retest at 18 months reactive HIV INFECTED

HIV TESTING POLICY IN PPTCT § PARENT: Informed consent of the patient Pre n post test counselling Routine testing with three rapid tests first: highly sensitive test-NR-reported with exception of WP Indeterminate: 1 st test reactive, 2 nd/3 rd NR repeat test after 14 -28 days. WB/PCR: For persistent indeterminate cases. § INFANT OF HIV + MOTHER, ASYMPTOMATIC: ELISA at 18 months—NR—Retest—NR—uninfected. Reactive—consider infected, confirm with retest. § INFANT OF HIV + MOTHER, SYMPTOMATIC: ELISA at 12 months—NR—retest NR—uninfected. Reactive—retest R at 18 months—infected.
