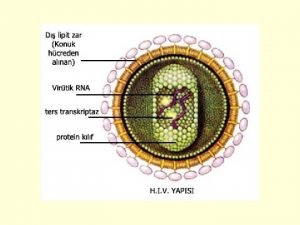
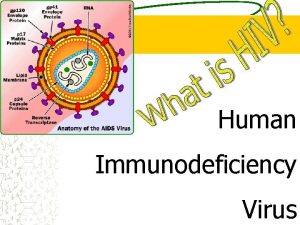
HIV Human Immunodeficiency Virus HIV is an RNA





















- Slides: 60

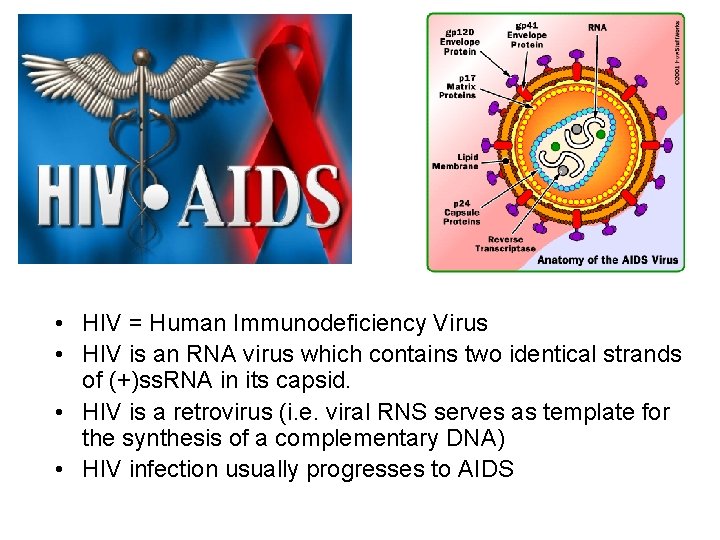
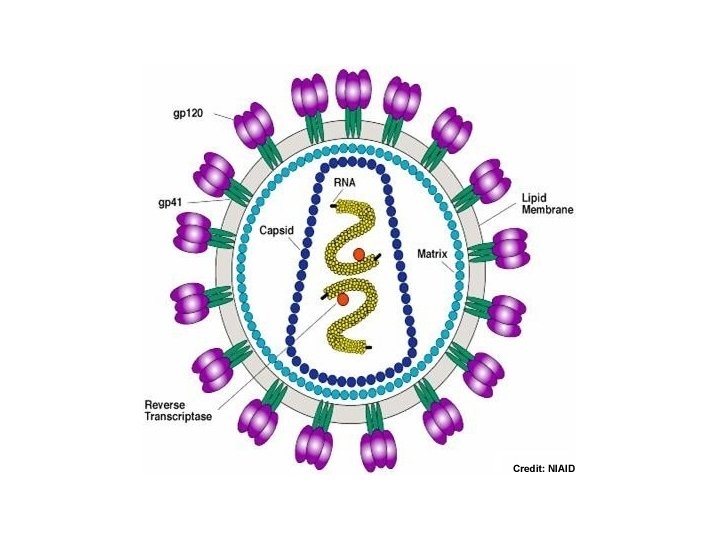
• HIV = Human Immunodeficiency Virus • HIV is an RNA virus which contains two identical strands of (+)ss. RNA in its capsid. • HIV is a retrovirus (i. e. viral RNS serves as template for the synthesis of a complementary DNA) • HIV infection usually progresses to AIDS

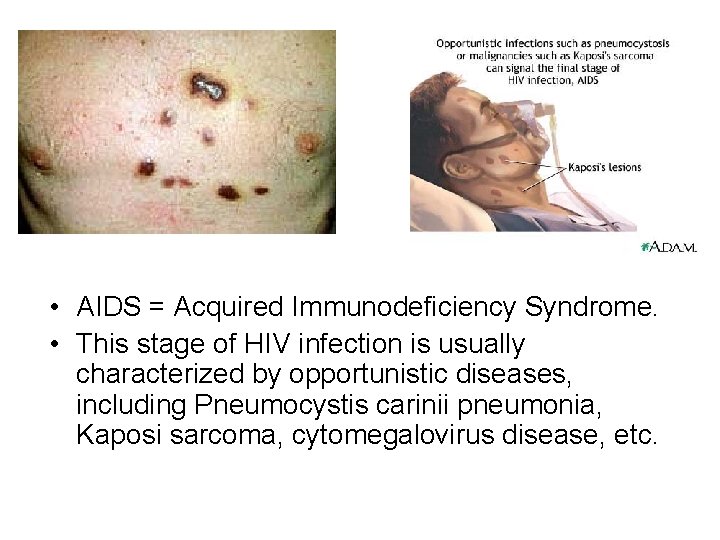
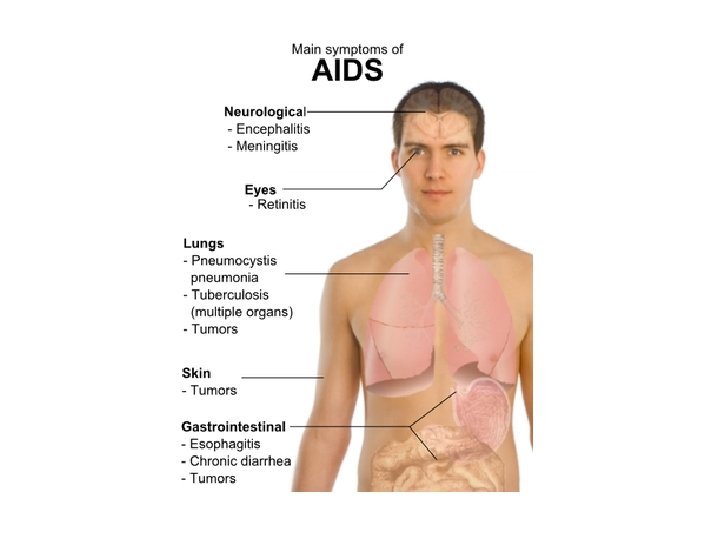
• AIDS = Acquired Immunodeficiency Syndrome. • This stage of HIV infection is usually characterized by opportunistic diseases, including Pneumocystis carinii pneumonia, Kaposi sarcoma, cytomegalovirus disease, etc.


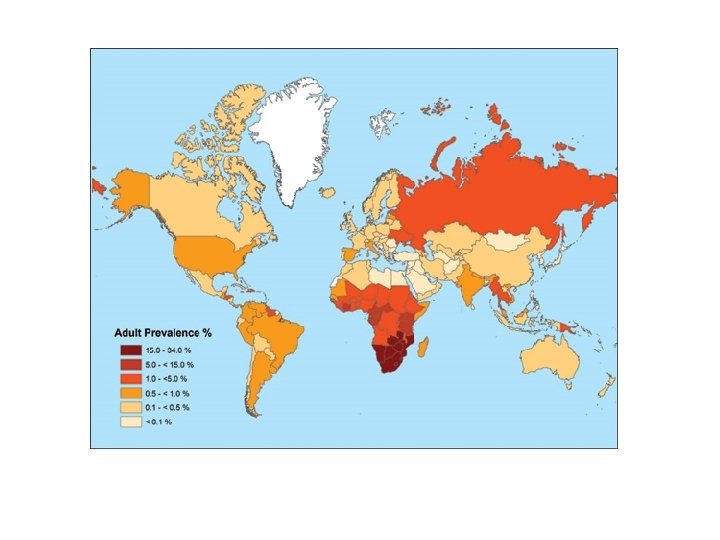
• HIV-1 is responsible for AIDS in America, Europe, and Asia • HIV-2 occurs mainly in western Africa • At present, anti-HIV drugs are aimed at two targets: reverse transcriptase and HIV protease.


• Good animation of HIV-1 Lifecycle: • http: //www. sumanasinc. com/webconten t/animations/content/lifecyclehiv. html • Link




Introduction to HIV treatment: Resistance • http: //biocreations. com/animations/engli sh_HIV/main. swf

HIV Lifecycle and Opportunities for New Therapeutic Agents • http: //www. rochehiv. com/portal/eipf/pb/hiv/Roche. HIV/demonstrationoffusioninhibition

Treatment of HIV • When HIV replicates (makes new copies of itself) it often makes mistakes. • Taking two or more antiretrovirals at the same time vastly reduces the rate at which resistance develops • The term Highly Active Antiretroviral Therapy (HAART) is used to describe a combination of three or more anti-HIV drugs.

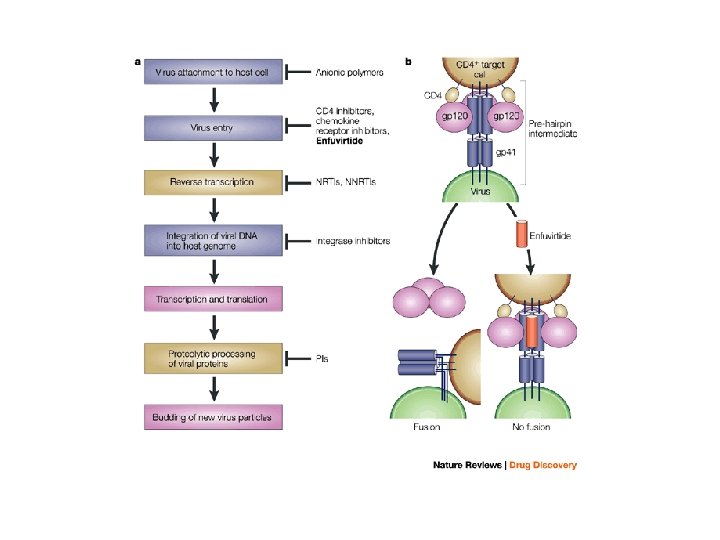
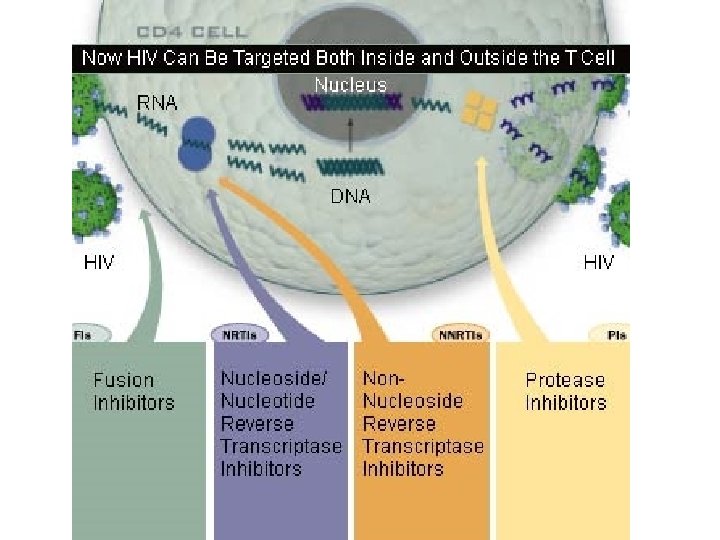
Treatment of HIV • Current classes of antiretroviral drugs include: – Nucleoside/Nucleotide Reverse Transcriptase Inhibitors – Non-Nucleoside Reverse Transcriptase Inhibitors – Protease Inhibitors – Fusion or Entry Inhibitors – Integrase Inhibitors

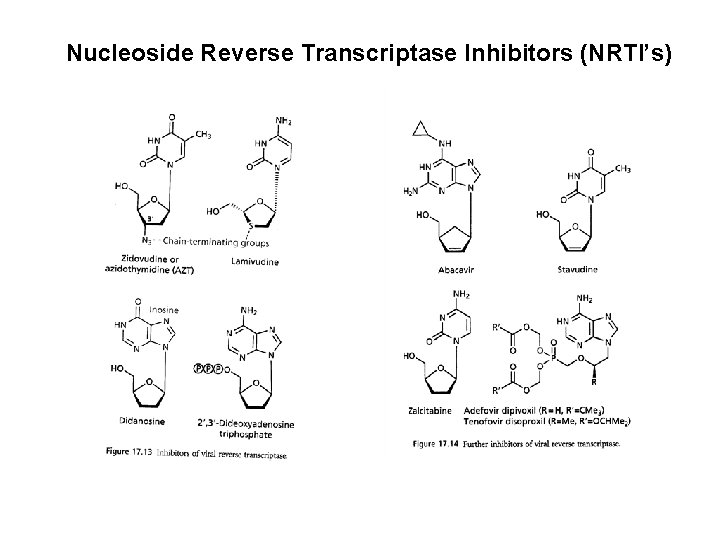
Nucleoside/Nucleotide Reverse Transcriptase Inhibitors • These were the first type of drug available to treat HIV infection in 1987. • NRTIs (also known as nucleoside analogues or nukes) interfere with the action of an HIV protein called reverse transcriptase, which the virus needs to make new copies of itself. • NRTIs are sometimes called the "backbone" of combination therapy because most regimens contain at least two of these drugs.

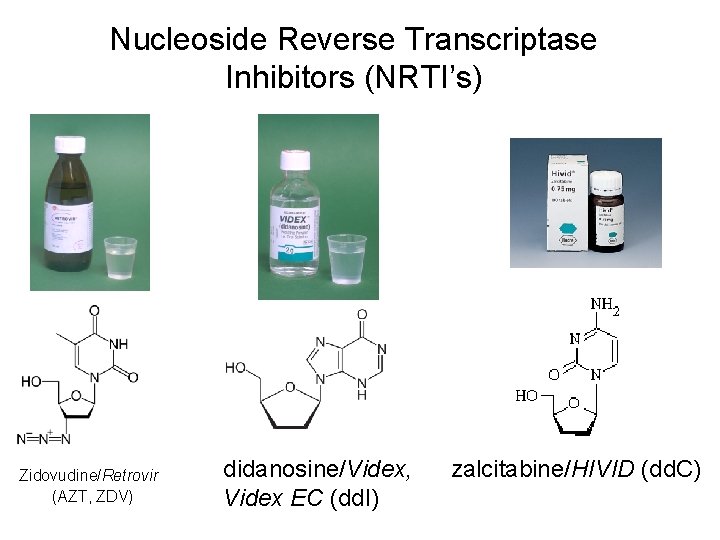
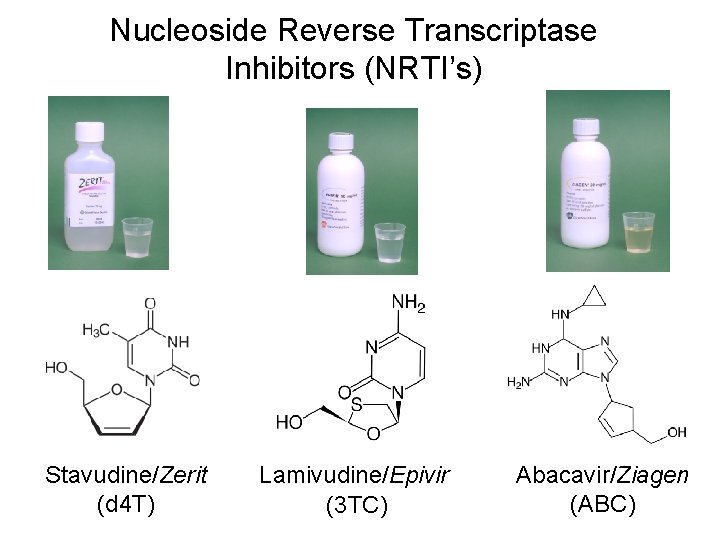
Antiretroviral Agents Currently Available (generic name/Trade name) Nucleoside Analogs (NRTI’s) • • • zidovudine/Retrovir(AZT, ZDV) didanosine/Videx, Videx EC (dd. I) zalcitabine/HIVID (dd. C) stavudine/Zerit (d 4 T) lamivudine/Epivir (3 TC) abacavir/Ziagen (ABC)

Nucleoside Reverse Transcriptase Inhibitors (NRTI’s) Zidovudine/Retrovir (AZT, ZDV) didanosine/Videx, Videx EC (dd. I) zalcitabine/HIVID (dd. C)

Nucleoside Reverse Transcriptase Inhibitors (NRTI’s) Stavudine/Zerit (d 4 T) Lamivudine/Epivir (3 TC) Abacavir/Ziagen (ABC)

Nucleoside Reverse Transcriptase Inhibitors (NRTI’s)

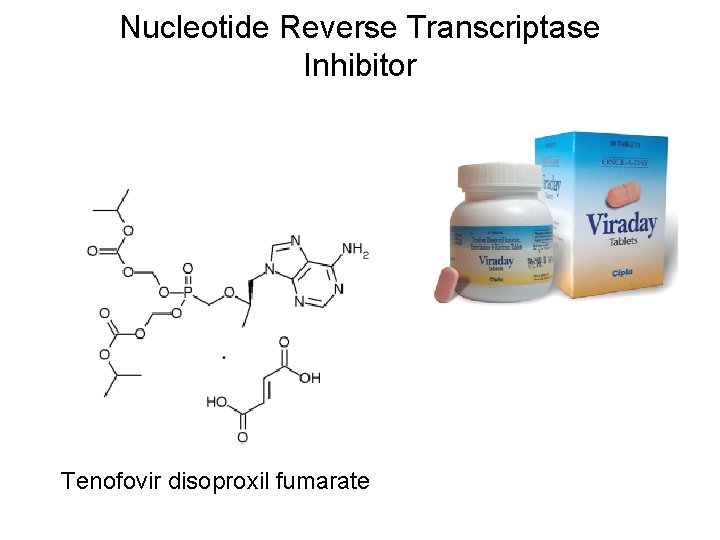
Nucleotide Reverse Transcriptase Inhibitor Tenofovir disoproxil fumarate

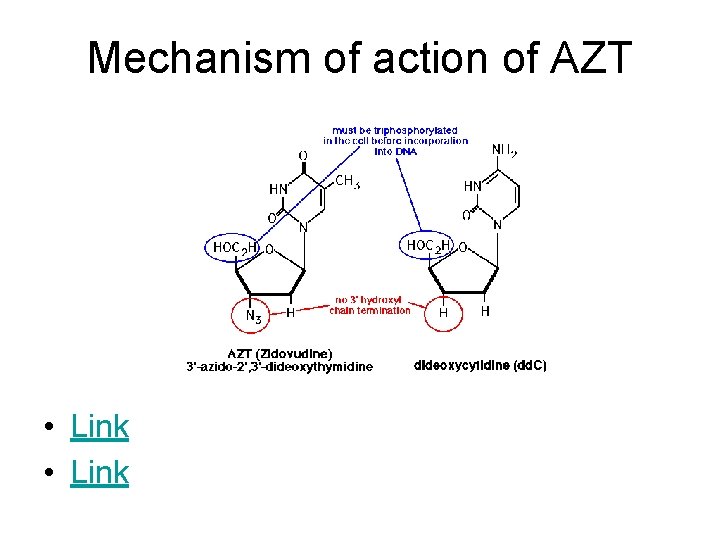
Mechanism of action of AZT • Link

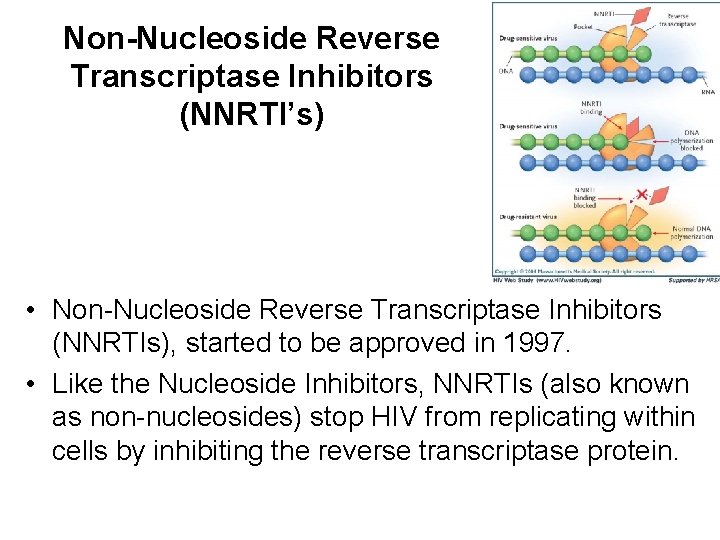
Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTI’s) • Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs), started to be approved in 1997. • Like the Nucleoside Inhibitors, NNRTIs (also known as non-nucleosides) stop HIV from replicating within cells by inhibiting the reverse transcriptase protein.

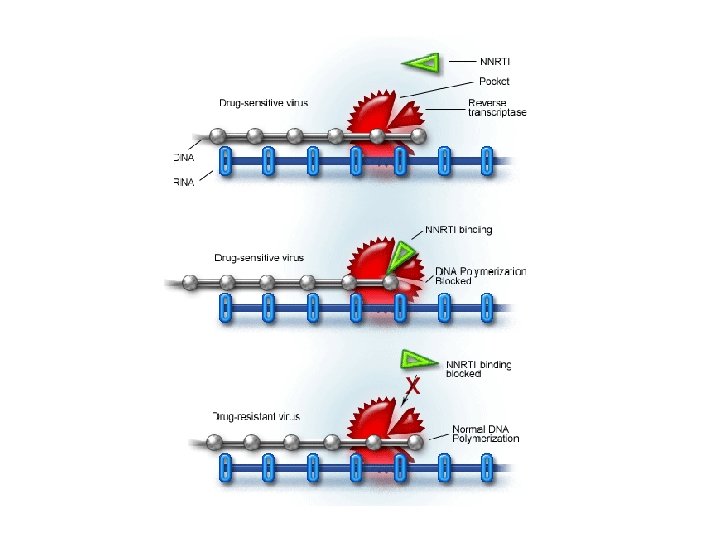
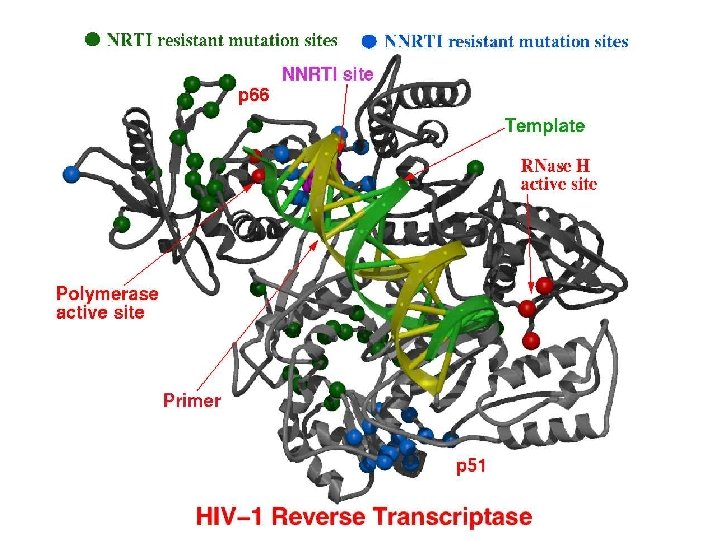
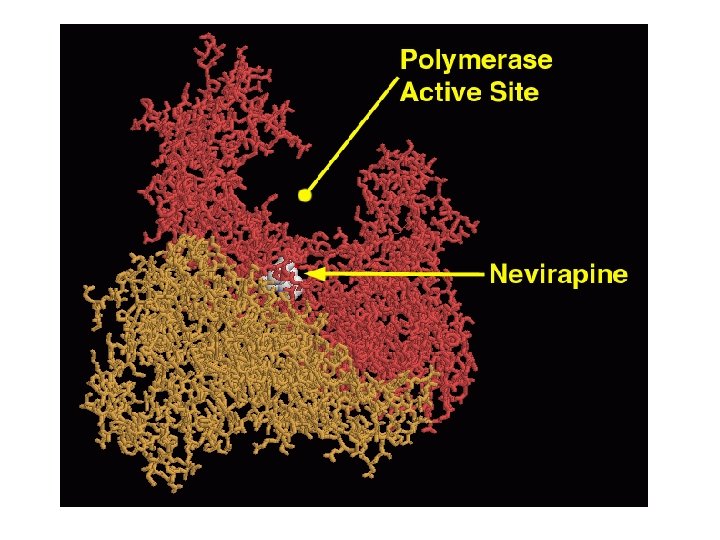
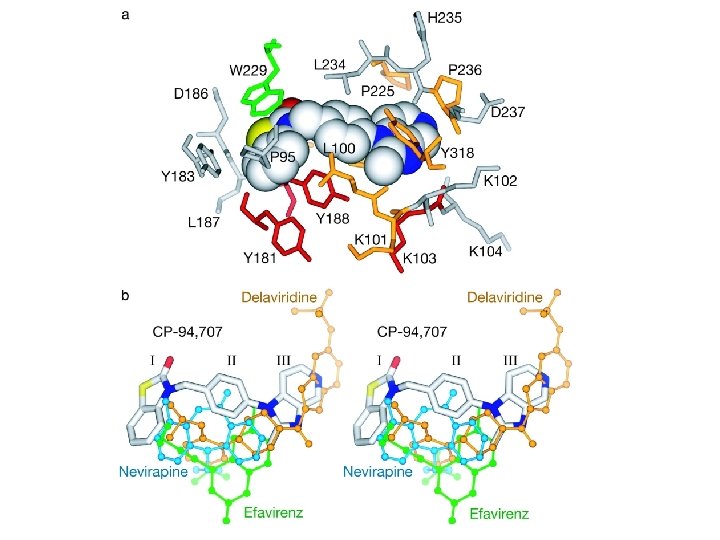
Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTI’s) • nevirapine/Viramune (NVP) • delavirdine/Rescriptor (DLV) • efavirenz/Sustiva (EFV) • NNRTI’s are generally hydrophobic molecules that bind to an allosteric binding site • Binding to this allosteric site locks the neighboring substrate-binding site into an inactive conformation. • However, resistance to NNRTI’s can develop rapidly, and thus they are used in combination with NRTI’s • Link



Non-nucleoside reverse transcriptase inhibitors



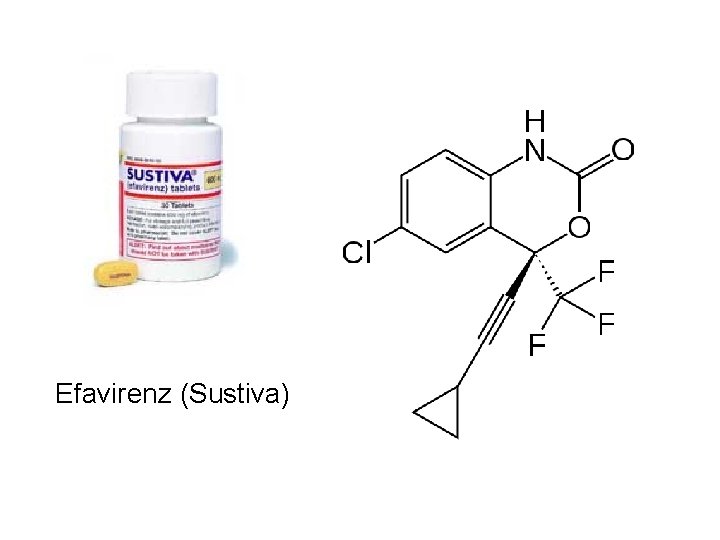
Efavirenz (Sustiva)


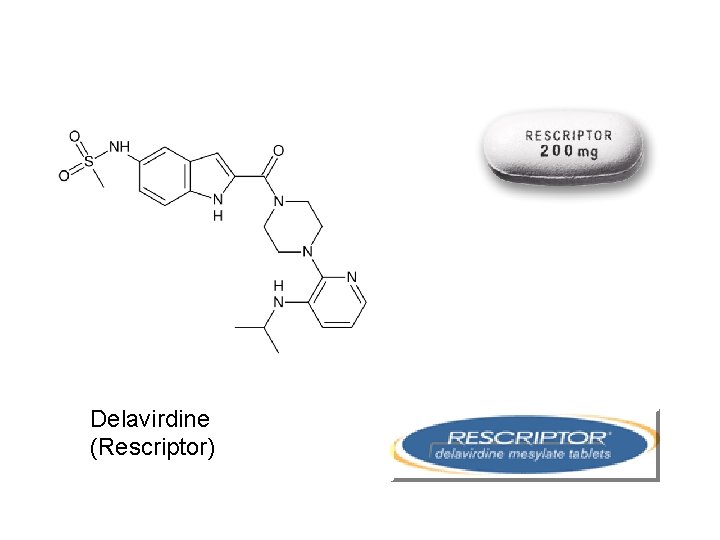
Delavirdine (Rescriptor)

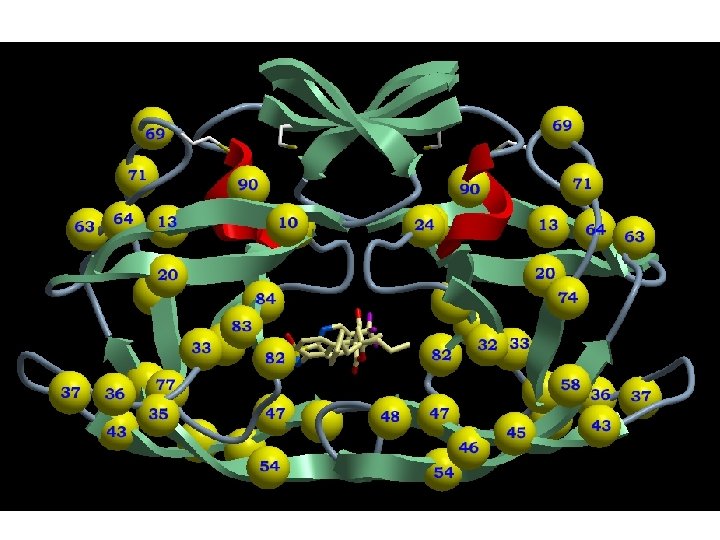
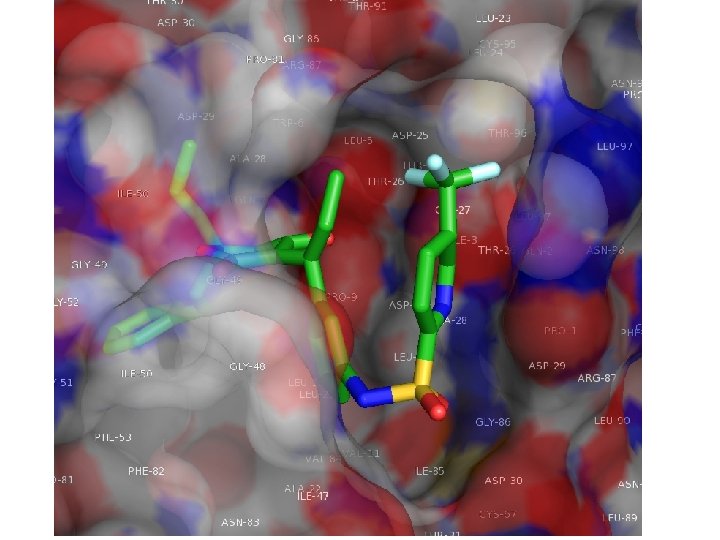
Protease Inhibitors • • • indinavir/Crixivan ritonavir/Norvirs aquinavir/Invirase, Fortovase nelfinavir/Viracept amprenavir/Ageneras elopinavir/ritonavir, Kaletra

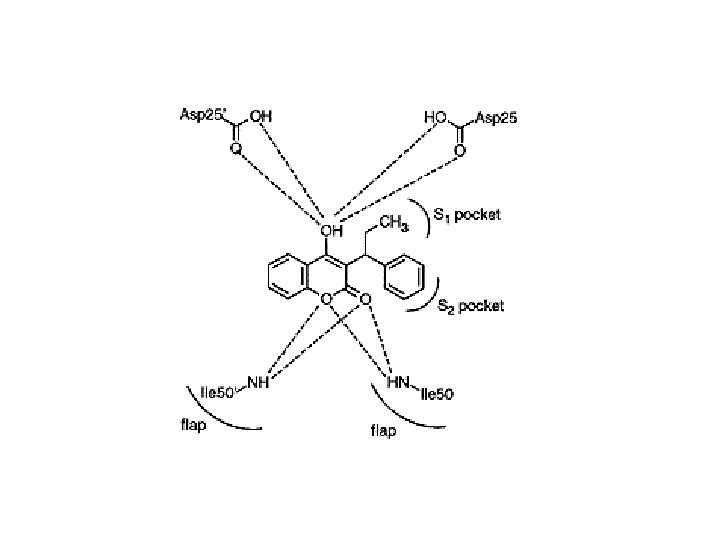
Chemical Mechanism of HIV Protease Hydrolysis Link

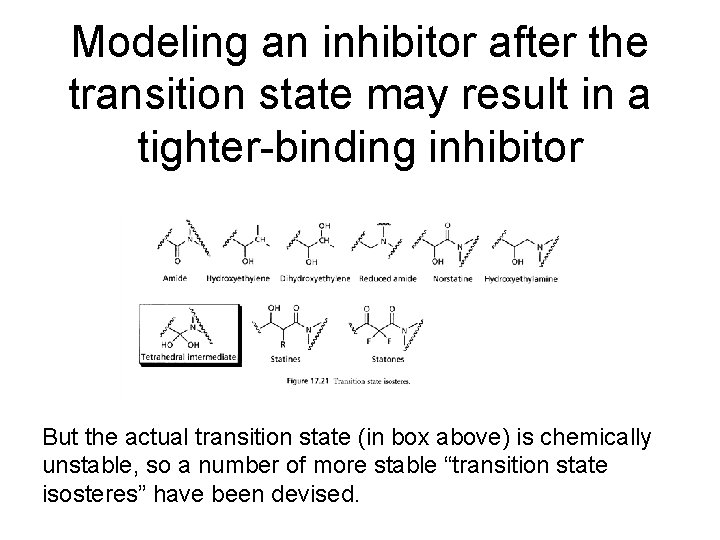
Modeling an inhibitor after the transition state may result in a tighter-binding inhibitor But the actual transition state (in box above) is chemically unstable, so a number of more stable “transition state isosteres” have been devised.

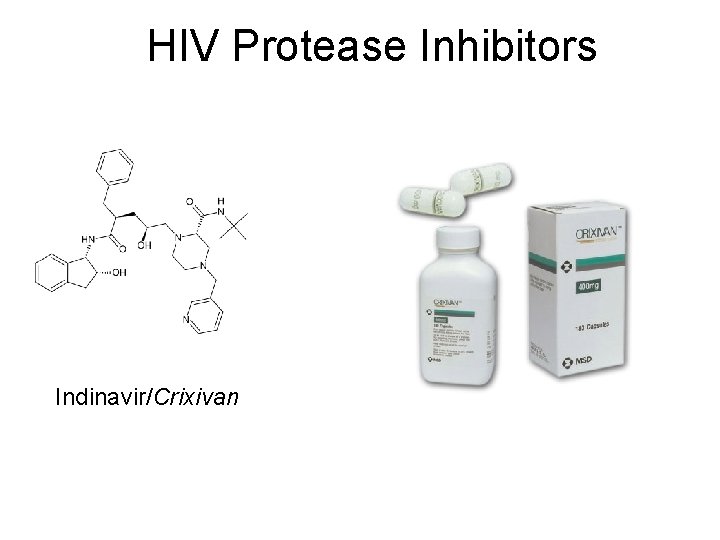
HIV Protease Inhibitors Indinavir/Crixivan

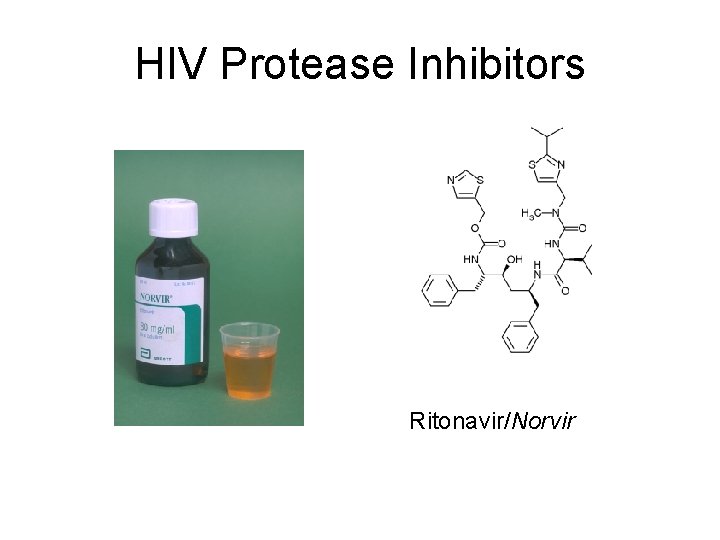
HIV Protease Inhibitors Ritonavir/Norvir

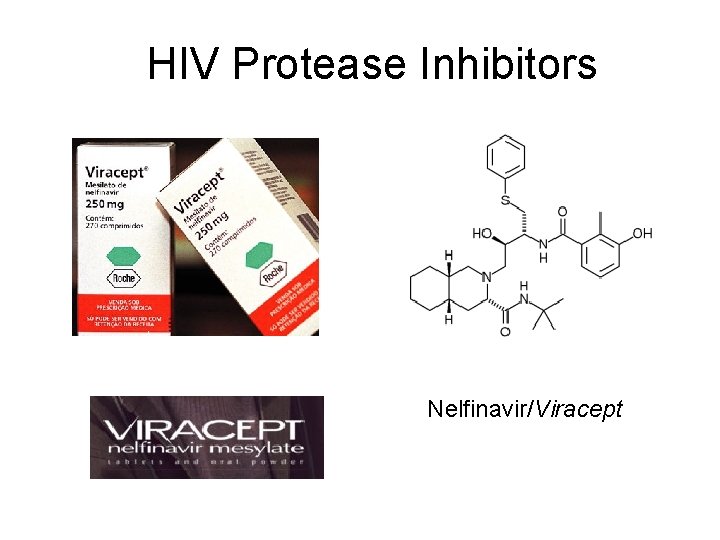
HIV Protease Inhibitors Nelfinavir/Viracept


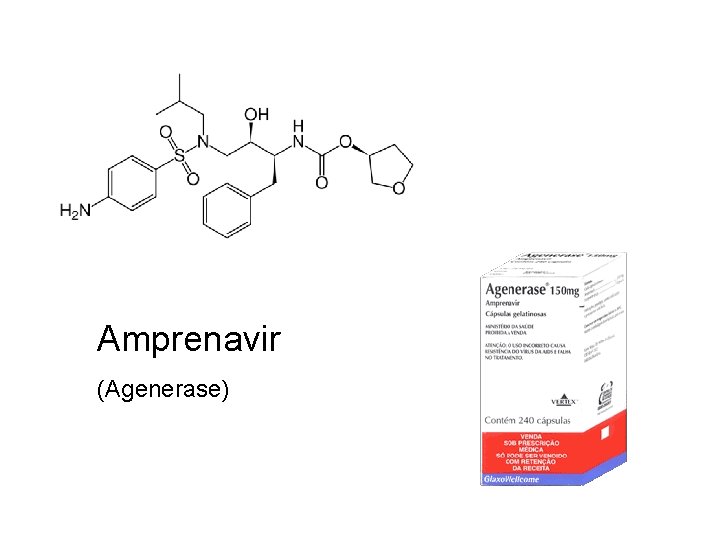
Amprenavir (Agenerase)

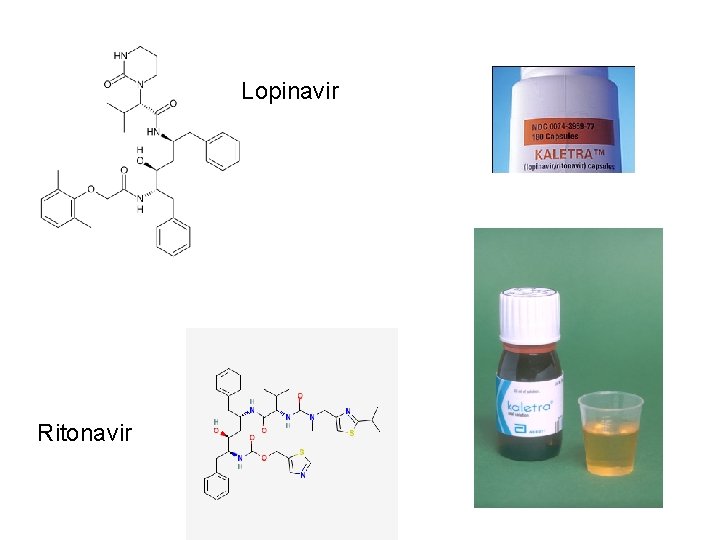
Lopinavir Ritonavir

Development of saquinavir

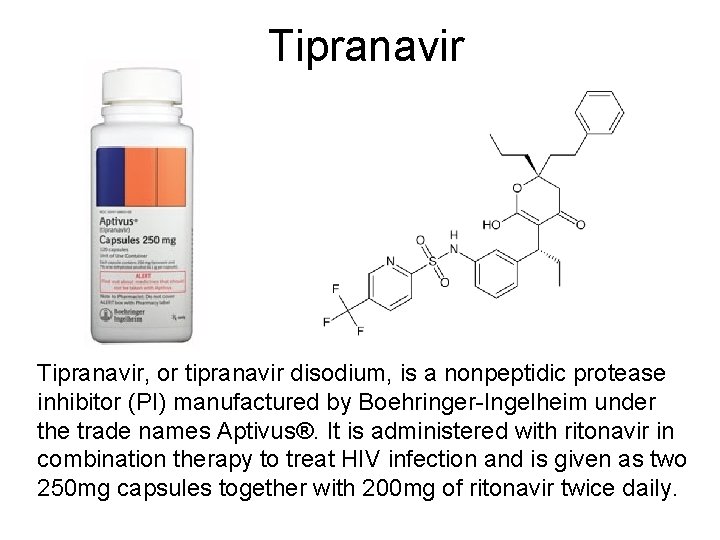
Tipranavir, or tipranavir disodium, is a nonpeptidic protease inhibitor (PI) manufactured by Boehringer-Ingelheim under the trade names Aptivus®. It is administered with ritonavir in combination therapy to treat HIV infection and is given as two 250 mg capsules together with 200 mg of ritonavir twice daily.

Tipranvir • Tipranavir has the ability to inhibit the replication of viruses that are resistant to other protease inhibitors and it recommended for patients who are resistant to other treatments. Resistance to tipranavir itself seems to require multiple mutations.

Animation of tipranavir, a new HIV protease inhibitor • http: //biosingularity. wordpress. com/200 7/07/04/super-3 d-animation-that-showsthe-mode-of-action-of-an-hiv-drug




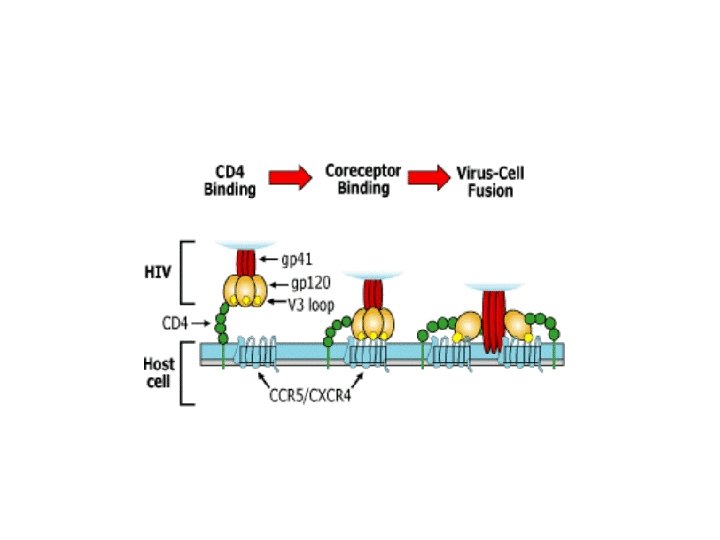
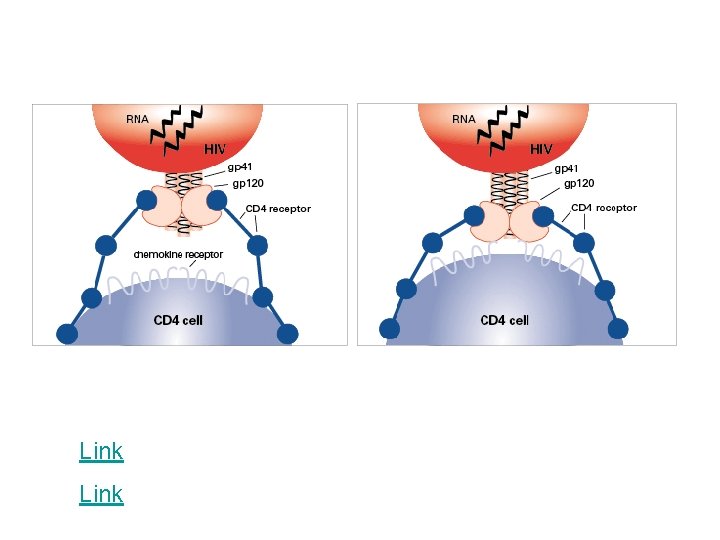
Fusion or Entry Inhibitors • Entry inhibitors prevent HIV from entering human immune cells. • There are several key proteins involved in the HIV entry process: – CD 4, a protein receptor found on the surface of Helper T cells in the human immune system, also called CD 4+ T cells – gp 120, a protein on HIV surface that binds to the CD 4 receptor – CCR 5, a second receptor found on the surface of CD 4+ cells, called a chemokine coreceptor – CXCR 4, another chemokine coreceptor found on CD 4+ cells – gp 41, a HIV protein, closely associated with gp 120, that penetrates the cell membrane

Link

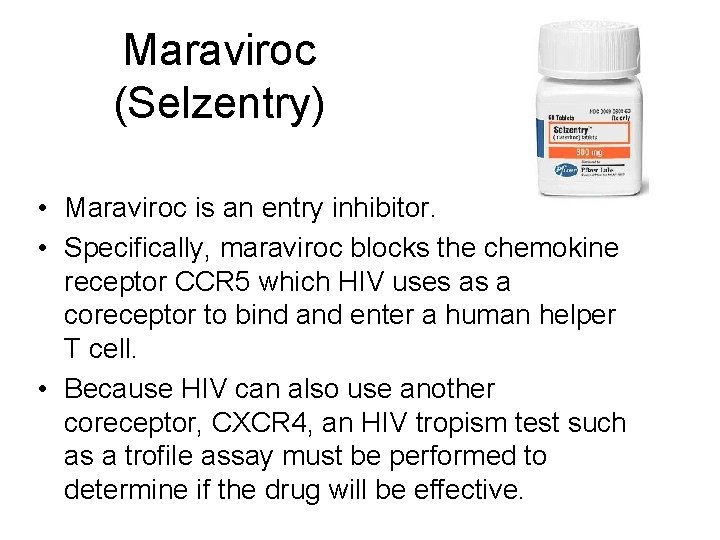
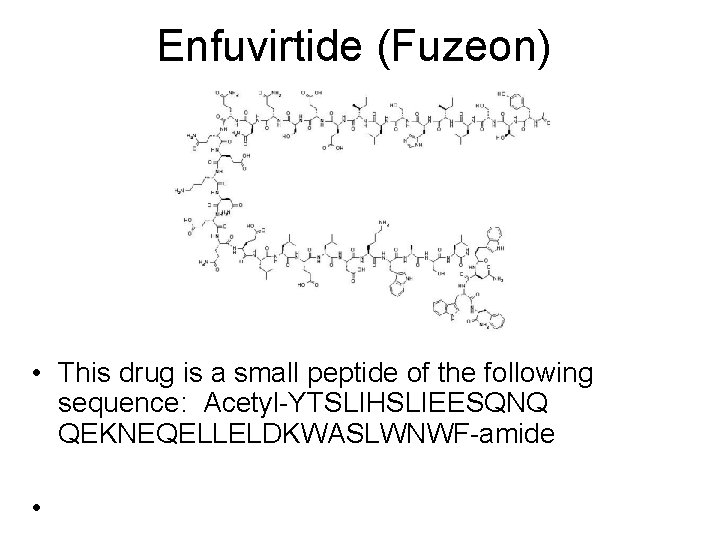
Approved Entry Inhibitors • Maraviroc (brand-named Selzentry, or Celsentri outside the U. S. ) • Enfuvirtide (INN) is an HIV fusion inhibitor, It is marketed under the trade name Fuzeon (Roche).

Maraviroc • Approved in April, 2007 and marketed by Pfizer

Maraviroc (Selzentry) • Maraviroc is an entry inhibitor. • Specifically, maraviroc blocks the chemokine receptor CCR 5 which HIV uses as a coreceptor to bind and enter a human helper T cell. • Because HIV can also use another coreceptor, CXCR 4, an HIV tropism test such as a trofile assay must be performed to determine if the drug will be effective.

Enfuvirtide (Fuzeon) • This drug is a small peptide of the following sequence: Acetyl-YTSLIHSLIEESQNQ QEKNEQELLELDKWASLWNWF-amide •


• By virtue of its peptide nature, enfuvirtide is marketed in injectable form. The lyophilised enfuvirtide powder must be reconstituted by the patient and administered twice daily by subcutaneous injection

Enfuvirtide (Fuzeon) • Enfuvirtide therapy costs an estimated $25, 000 per year in the United States. • Its cost and inconvenient dosing regimen are factors behind its use as a reserve, for "salvage" therapy in patients with multi-drug resistant HIV.

Approved HIV Integrase Inhibitor • Raltegravir (MK-0518, brand name Isentress. TM) is an antiretroviral drug produced by Merck & Co, used to treat HIV infection. • It received FDA approval in October 2007, the first of a new class of HIV drugs, the integrase inhibitors, to receive such approval.

Raltegravir (Isentress) • Raltegravir is approved only for use only in individuals whose infection has proven resistant to other HAART drugs. • As with any HAART medication, raltegravir is unlikely to show durability if used as monotherapy. • Raltegravir is taken orally twice daily.


Assigned Reading • An Introduction to Medicinal Chemistry by Graham L. Patrick, pp. 440 -486.

Homework Questions • Draw the structure of AZT and discuss how the nucleoside reverse transcriptase inhibitors (NRTIs) interfere with DNA synthesis. Structurally, what must happen to these molecules before they can perform their function? • Show the stepwise mechanism for the hydrolysis of a peptide bond catalyzed by an aspartyl protease (such as HIV protease) using arrows to depict the movement of electrons. • Draw the structure of saquinavir, the first HIV protease inhibitor on the market, and discuss how/why this inhibitor is effective against this viral enzyme.
 Monocyte derived dendritic cells
Monocyte derived dendritic cells Severe combined immunodeficiency
Severe combined immunodeficiency Stadium hiv
Stadium hiv Hiv virus
Hiv virus Picornavirus adalah
Picornavirus adalah Rna virus
Rna virus Virusmax
Virusmax Rna virus
Rna virus Corona virus dna or rna
Corona virus dna or rna Quang trung
Quang trung Hiv reverse transcription
Hiv reverse transcription Test wiedzy o aids z odpowiedziami
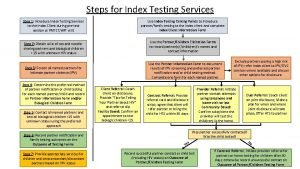
Test wiedzy o aids z odpowiedziami Approche index testing
Approche index testing Elemen penilaian prognas
Elemen penilaian prognas Phdp in hiv
Phdp in hiv Kuchecheudzwa
Kuchecheudzwa Window period hiv
Window period hiv Dot
Dot Iris hiv
Iris hiv Hiv
Hiv Hiv risk factors
Hiv risk factors Asante hiv-1 rapid recency assay
Asante hiv-1 rapid recency assay Fiebig hiv
Fiebig hiv Hiv case-based surveillance in ethiopia
Hiv case-based surveillance in ethiopia Triệu chứng nhiễm hiv
Triệu chứng nhiễm hiv A bacterial std that usually affects mucous membranes
A bacterial std that usually affects mucous membranes Chapter 24 sexually transmitted diseases and hiv/aids
Chapter 24 sexually transmitted diseases and hiv/aids Basic hiv course
Basic hiv course Stakeholders in hiv prevention
Stakeholders in hiv prevention Hiv treatments
Hiv treatments Causative organism of hiv/aids
Causative organism of hiv/aids What does hiv stand
What does hiv stand What does hiv stand
What does hiv stand Hiv test window period
Hiv test window period Hiv stool color
Hiv stool color Procentowe ryzyko zakażenia hiv
Procentowe ryzyko zakażenia hiv Leukopenia
Leukopenia Hiv lifecycle
Hiv lifecycle Hiv pencere dönemi
Hiv pencere dönemi Hiv lifecycle
Hiv lifecycle Hiv
Hiv Chapter 17 oral pathology
Chapter 17 oral pathology Incubation period of hiv
Incubation period of hiv Imune
Imune Vidas hiv duo ultra package insert
Vidas hiv duo ultra package insert Profilaksis pasca pajanan hiv
Profilaksis pasca pajanan hiv Igéje szól igéje hív
Igéje szól igéje hív Hiv patologia
Hiv patologia Cytomegalavirus
Cytomegalavirus Hiv siv
Hiv siv Syntom på klamydia
Syntom på klamydia Hiv roga lakshana
Hiv roga lakshana Negative hiv test result
Negative hiv test result Hiv
Hiv Hiv
Hiv Hiv
Hiv Hiv life cycle
Hiv life cycle Ciclo do hiv
Ciclo do hiv Phagocytosis ap bio
Phagocytosis ap bio Hiv siv
Hiv siv Hiv stays alive in dried blood
Hiv stays alive in dried blood