History of the Periodic Table John Newland Law
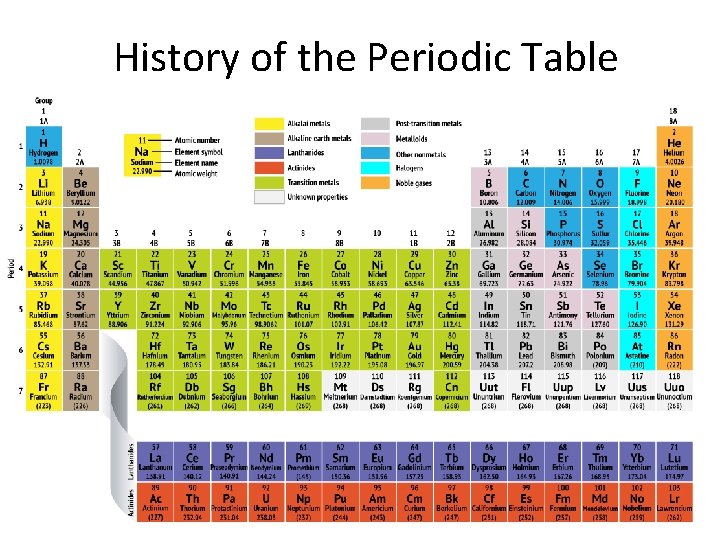
History of the Periodic Table
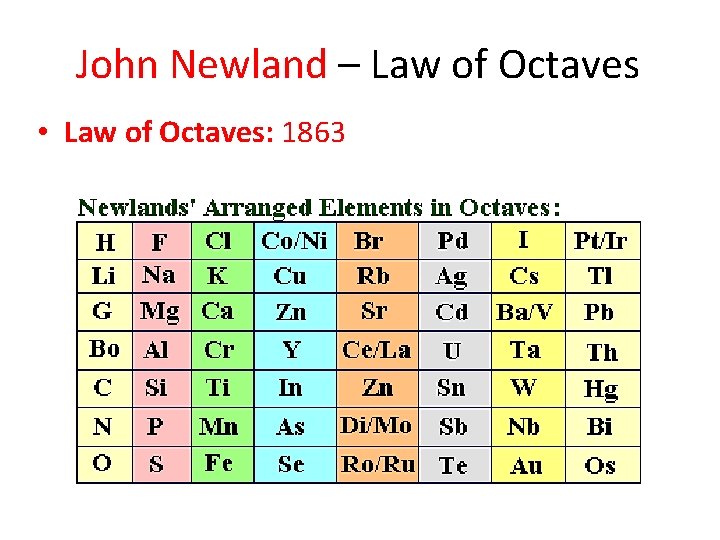
John Newland – Law of Octaves • Law of Octaves: 1863
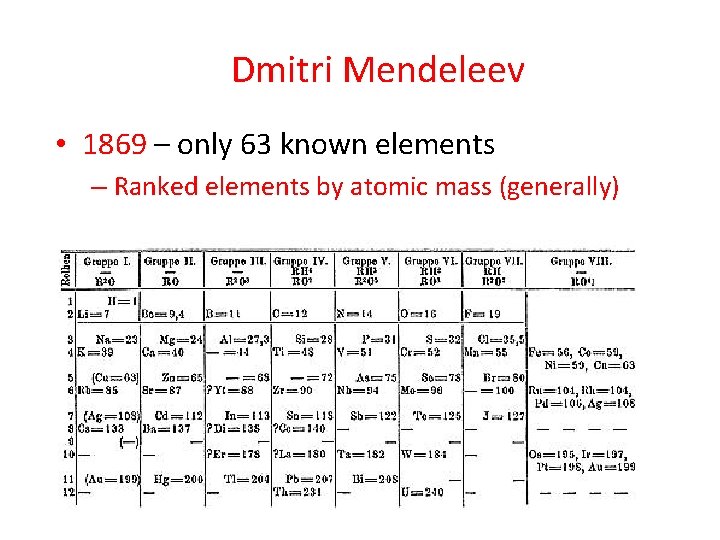
Dmitri Mendeleev • 1869 – only 63 known elements – Ranked elements by atomic mass (generally)
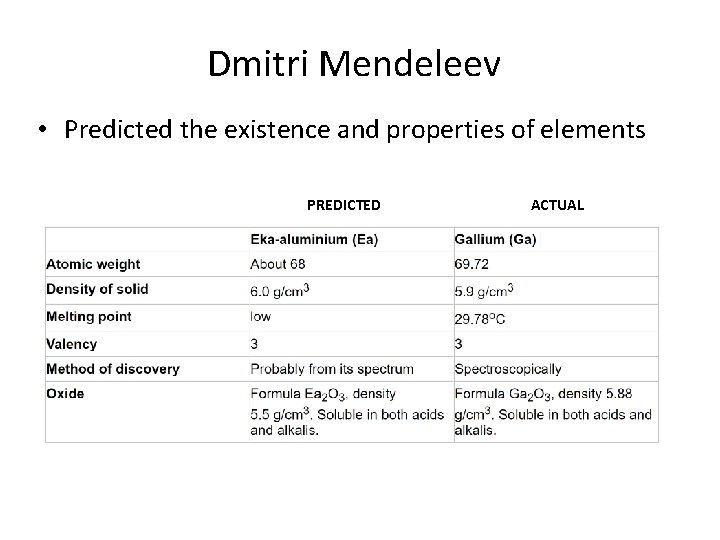
Dmitri Mendeleev • Predicted the existence and properties of elements PREDICTED ACTUAL

Lord Rayleigh – Noble Gases • 1895 – first noble gas discovered. – Argon – nonreactive. Placed at the end of the table. He Ne Kr Ar Xe
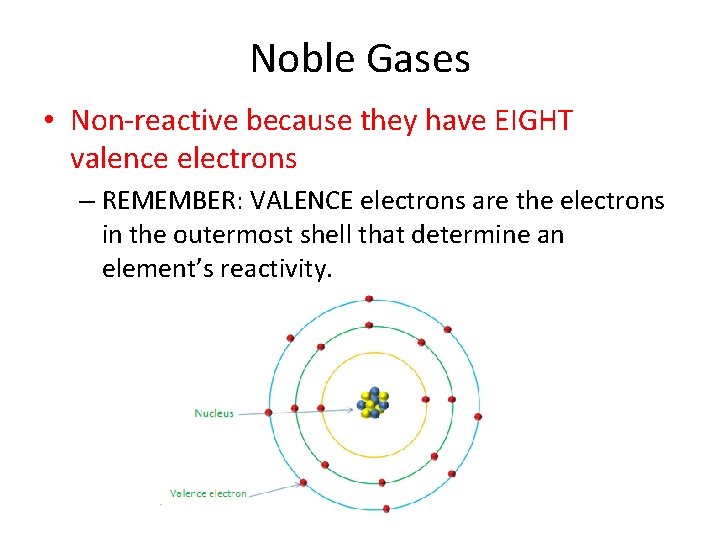
Noble Gases • Non-reactive because they have EIGHT valence electrons – REMEMBER: VALENCE electrons are the electrons in the outermost shell that determine an element’s reactivity.
Octet Rule • The octet rule states that elements gain or lose electrons until they have an electron configuration like the nearest noble gas. Elements and compounds WANT to have a full outer electron shell.
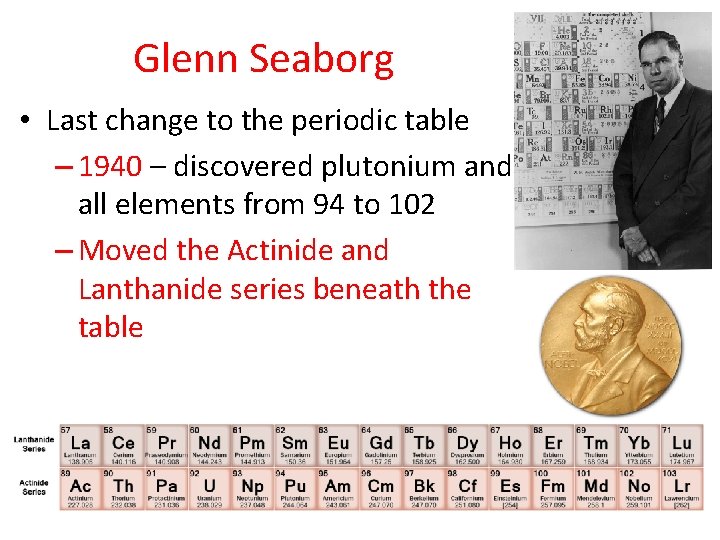
Glenn Seaborg • Last change to the periodic table – 1940 – discovered plutonium and all elements from 94 to 102 – Moved the Actinide and Lanthanide series beneath the table
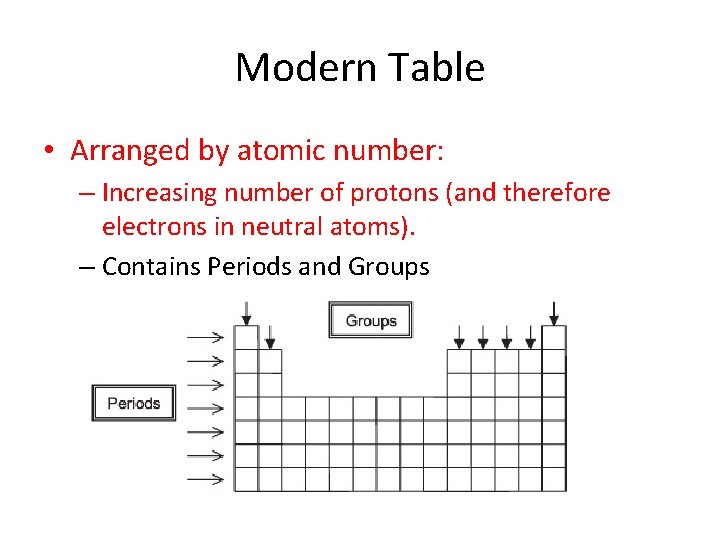
Modern Table • Arranged by atomic number: – Increasing number of protons (and therefore electrons in neutral atoms). – Contains Periods and Groups
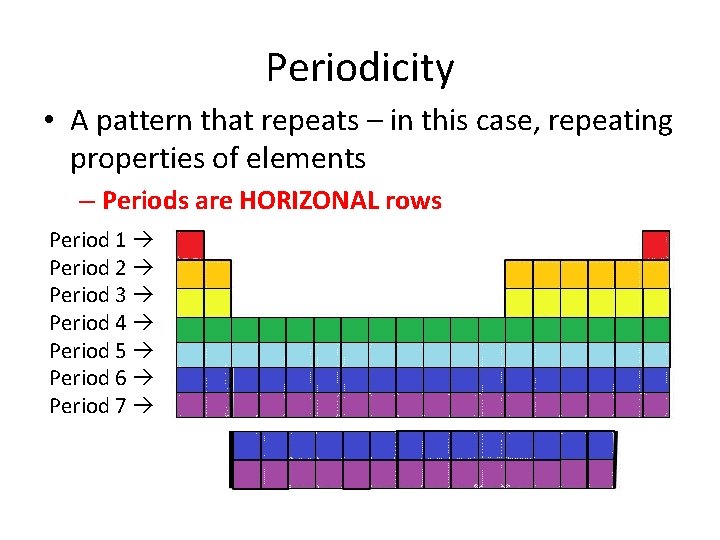
Periodicity • A pattern that repeats – in this case, repeating properties of elements – Periods are HORIZONAL rows Period 1 Period 2 Period 3 Period 4 Period 5 Period 6 Period 7
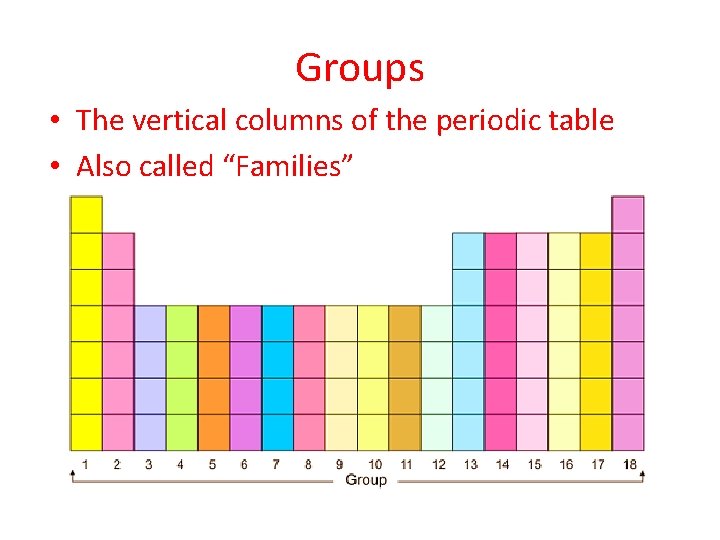
Groups • The vertical columns of the periodic table • Also called “Families”
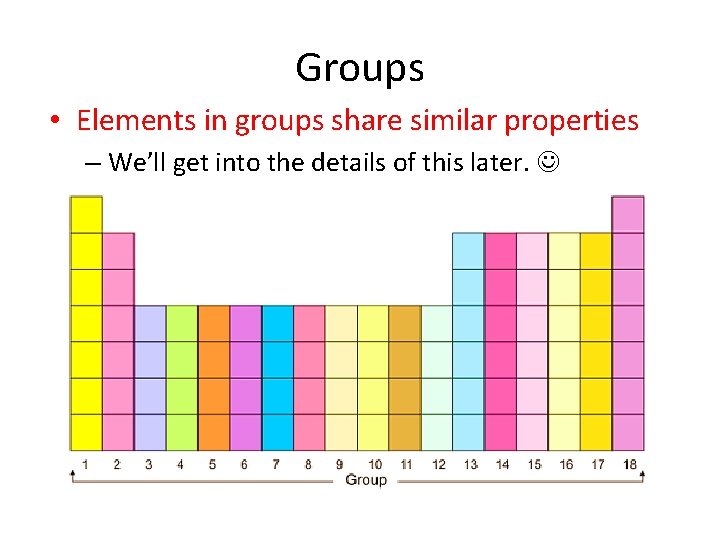
Groups • Elements in groups share similar properties – We’ll get into the details of this later.
Exit Slip Write down any questions that you have about the periodic table.
- Slides: 13