History of the Periodic Table Early 1800 s
History of the Periodic Table

Early 1800 s. Only about 30 known elements. Dobereiner observes that elements seem to belong in groups of three- Triads

Triads- groups of three elements with similar properties • Examples: Lithium (Li), Sodium (Na), and Potassium (K) Very reactive metals Chlorine, Bromine, Iodine Very reactive nonmetals Magnesium, Calcium, Strontium When looking at a single property, one element seemed to be an average of the other two

Atomic mass Lithium = 7 amu Potassium = 39 amu 7 + 39 = 46, 46/2 = 23 Sodium has an atomic mass of 23

Density Chlorine =1. 56 g/Liter Iodine = 4. 95 1. 56 + 4. 95 = 6. 51 , 6. 51 /2 = 3. 26 Bromine = 3. 12, which is close to 3. 26


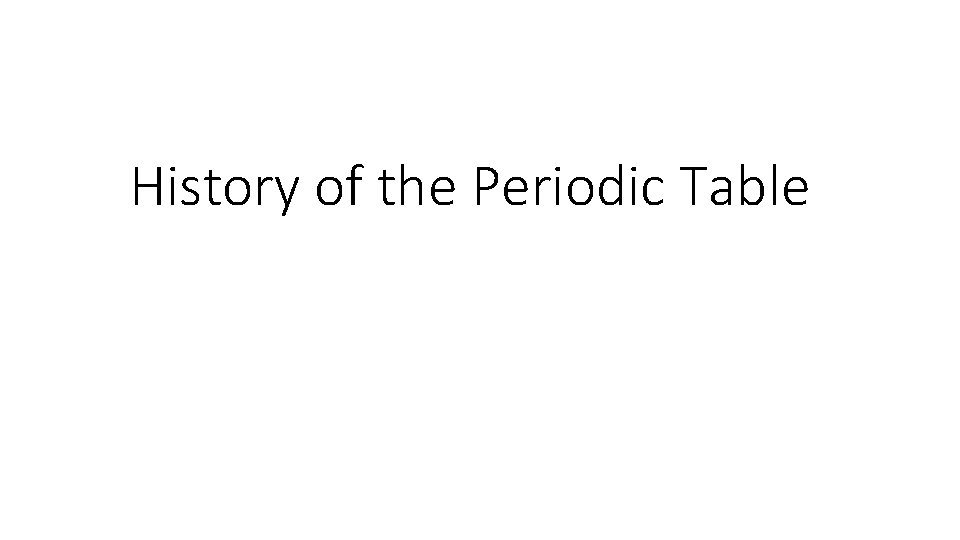
Newlands -1865 Law of Octaves If arranged by atomic mass, every eighth element has similar properties

Dimitri Mendeleev 1869 First periodic Table Periodic Law- If placed in increasing atomic mass, elements show a repeating pattern of properties


Mendeleev had two important features in his Periodic table. 1) He left three open spaces on his periodic table for undiscovered elements and correctly predicted their properties
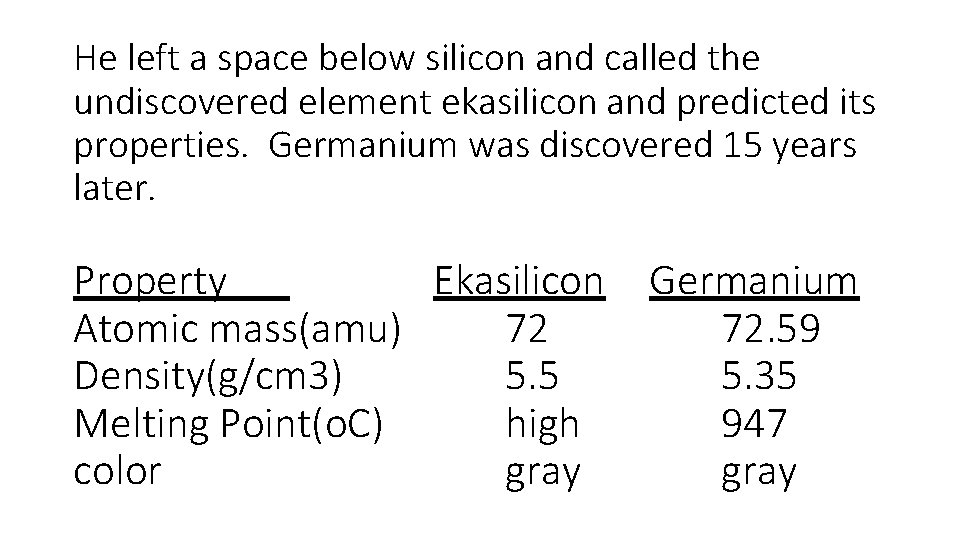
He left a space below silicon and called the undiscovered element ekasilicon and predicted its properties. Germanium was discovered 15 years later. Property Ekasilicon Atomic mass(amu) 72 Density(g/cm 3) 5. 5 Melting Point(o. C) high color gray Germanium 72. 59 5. 35 947 gray

He put some elements is spaces that didn’t follow his periodic law. Instead he placed them according to properties. Example: Argon (Ar) has a mass of 39. 948, Potassium (K) has a mass of 39. 0983. He placed Ar with the other Noble gases and K with the very reactive Alkali metals. “Someday, some one will figure out why? ”

Henry Moseley 1913 Discovered Protons and how to determine the atomic number of an atom Periodic law- If placed in order of atomic number, element show a repeating pattern of properties

Moseley was drafted into the British army and died in a trench in Germany. He is considered to be one of the brightest scientists ever.
- Slides: 15