History of the Atomic Model Democritus 400 BC
History of the Atomic Model

Democritus (~400 BC) • Greek philosopher who proposed that matter was made of tiny individual particles called “atomos” • Believed atoms were indivisible and indestructible • Approach was not based on the scientific method

Period 5 - Thursday, 10/22 Atomic Structure Test TOMORROW! Protons, neutrons, and electrons Average atomic mass calculations Atomic History- Names, experiments, models, timeline Use pgs. 9 -14 in packet for review questions key will be posted on shscience. net


John Dalton (1800) • Proposed that atoms are tiny, indestructible particles, with no internal structure • Known as the hard sphere model

Dalton’s Theory 1) Matter is made up of tiny, indivisible particles called atoms 2) All atoms of a given element are identical 3) Atoms of different elements have different properties and masses

Dalton’s Theory, cont. 4) Different atoms combine in whole number ratios to form compounds 5) In a chemical reaction, atoms are separated, rearranged, or combined- but never changed into atoms of another element

Sir William Crookes (1875)
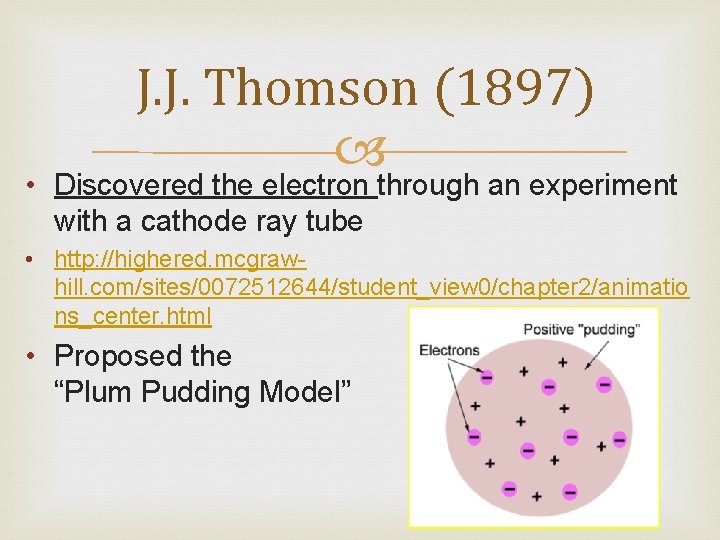
J. J. Thomson (1897) • Discovered the electron through an experiment with a cathode ray tube • http: //highered. mcgrawhill. com/sites/0072512644/student_view 0/chapter 2/animatio ns_center. html • Proposed the “Plum Pudding Model”

Robert Millikan (1909) • Performed the oil drop experiment to determine the charge on an electron (1. 602 x 10 -19 coulombs)

Ernest Rutherford (1911) • Performed the Gold Foil Experiment • Bombarded a thin piece of gold foil with a positive stream of alpha particles http: //www. mhhe. com/physsci/chemistry/animations/ chang_2 e/rutherfords_experiment. swf • 3 observations and 3 conclusions • Known as the nuclear model
- Slides: 11