HISTORY OF THE ATOM MODERN THEORIES OF ATOMIC
- Slides: 6

HISTORY OF THE ATOM MODERN THEORIES OF ATOMIC STRUCTURE

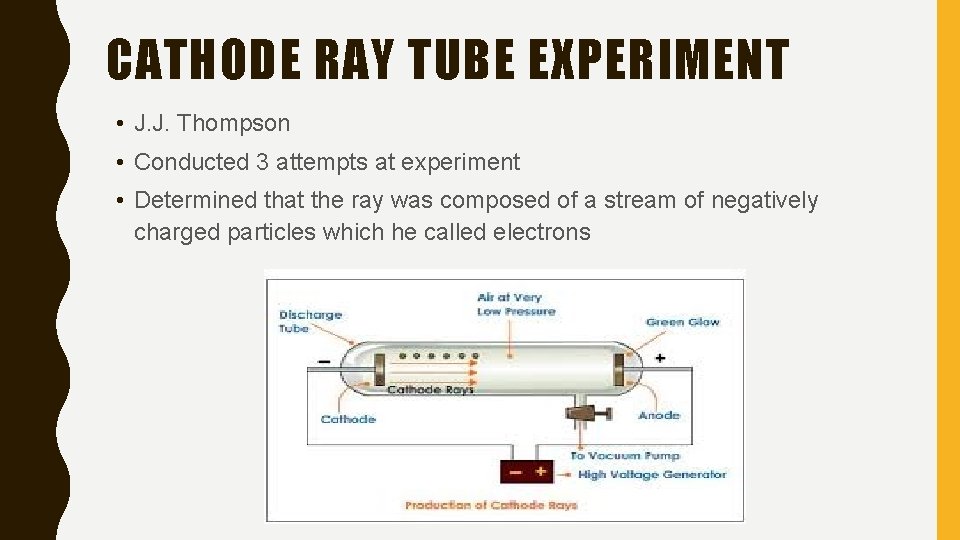
CATHODE RAY TUBE EXPERIMENT • J. J. Thompson • Conducted 3 attempts at experiment • Determined that the ray was composed of a stream of negatively charged particles which he called electrons

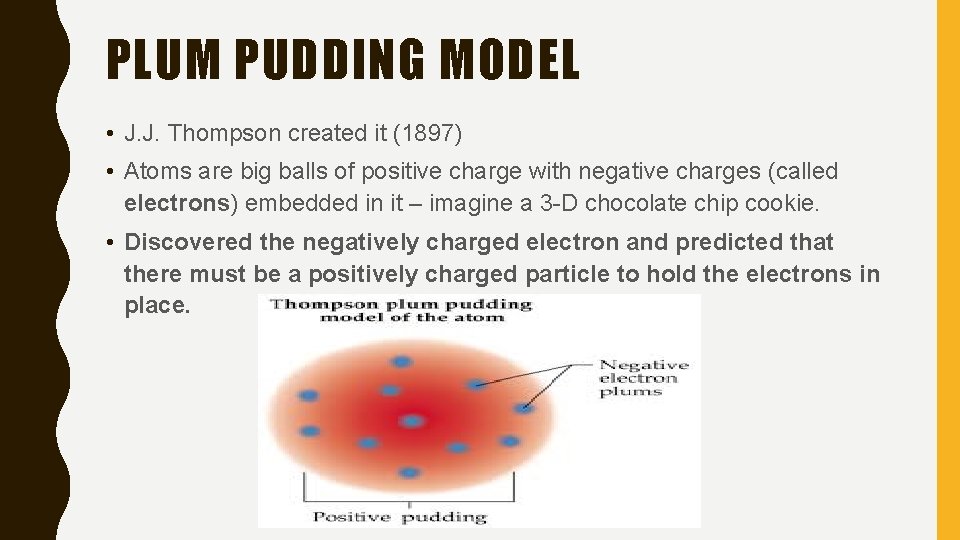
PLUM PUDDING MODEL • J. J. Thompson created it (1897) • Atoms are big balls of positive charge with negative charges (called electrons) embedded in it – imagine a 3 -D chocolate chip cookie. • Discovered the negatively charged electron and predicted that there must be a positively charged particle to hold the electrons in place.

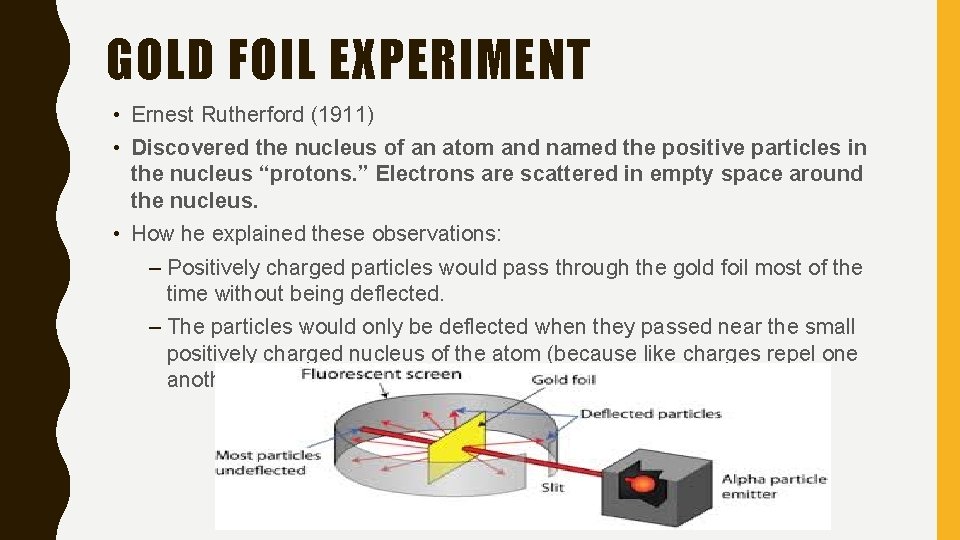
GOLD FOIL EXPERIMENT • Ernest Rutherford (1911) • Discovered the nucleus of an atom and named the positive particles in the nucleus “protons. ” Electrons are scattered in empty space around the nucleus. • How he explained these observations: – Positively charged particles would pass through the gold foil most of the time without being deflected. – The particles would only be deflected when they passed near the small positively charged nucleus of the atom (because like charges repel one another)

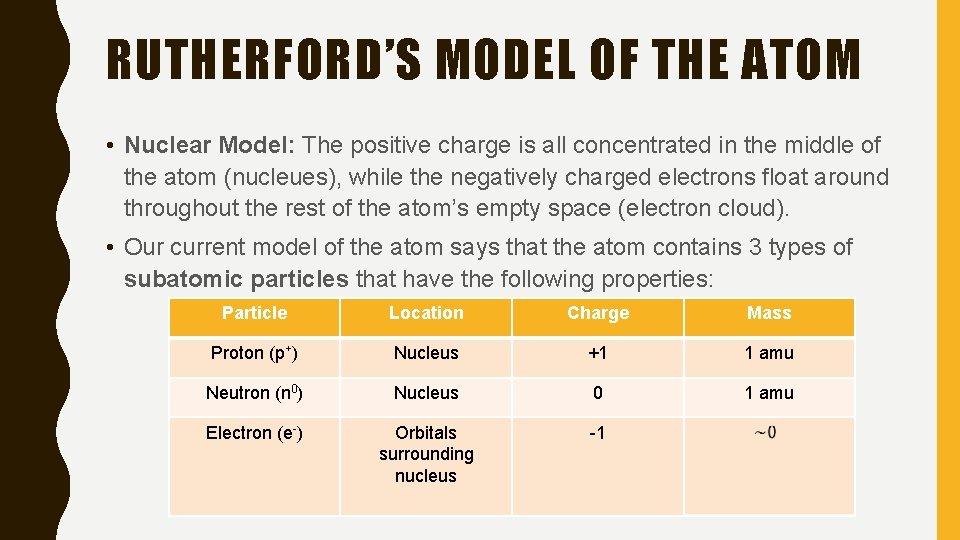
RUTHERFORD’S MODEL OF THE ATOM • Nuclear Model: The positive charge is all concentrated in the middle of the atom (nucleues), while the negatively charged electrons float around throughout the rest of the atom’s empty space (electron cloud). • Our current model of the atom says that the atom contains 3 types of subatomic particles that have the following properties: Particle Location Charge Mass Proton (p+) Nucleus +1 1 amu Neutron (n 0) Nucleus 0 1 amu Electron (e-) Orbitals surrounding nucleus -1

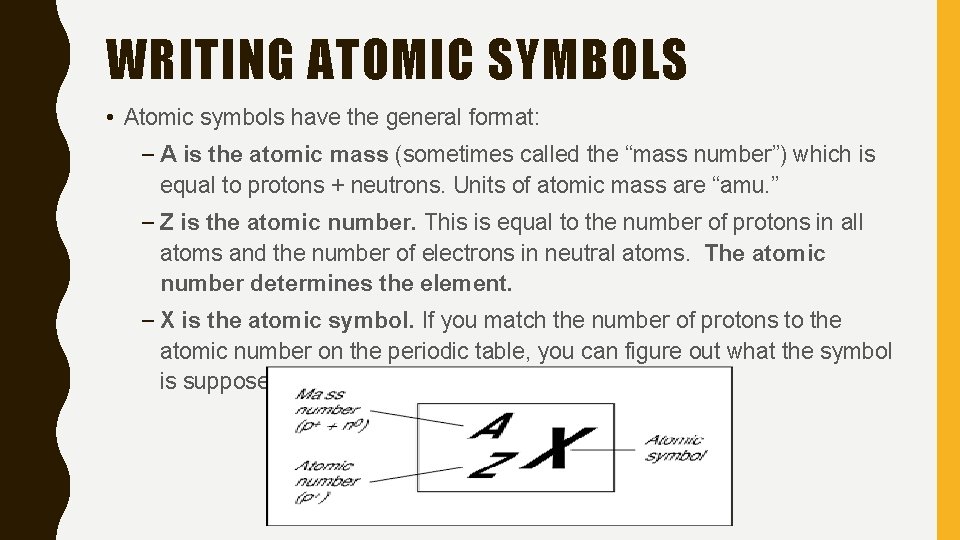
WRITING ATOMIC SYMBOLS • Atomic symbols have the general format: – A is the atomic mass (sometimes called the “mass number”) which is equal to protons + neutrons. Units of atomic mass are “amu. ” – Z is the atomic number. This is equal to the number of protons in all atoms and the number of electrons in neutral atoms. The atomic number determines the element. – X is the atomic symbol. If you match the number of protons to the atomic number on the periodic table, you can figure out what the symbol is supposed to be.