HISTORY OF THE ATOM Initially believed that all
HISTORY OF THE ATOM

Initially believed that all matter was composed of things such as earth, water, air and fire Also believed that matter could be endlessly divided EARLY PHILOSOPHERS
Greek Philosopher (460 -370 B. C. ) Believed matter was made up of tiny particles called atomos (atoms) Stated atom was the smallest particle and could not be divided Different sizes and shapes of different atoms DEMOCRITUS

English schoolteacher (1766 -1844) Performed multiple experiments, studied numerous chemical reactions and determined mass ratios to help support his theory Several ideas in Dalton’s Atomic Theory Matter is composed of extremely small particles called atoms Atoms are indivisible ** Atoms of a given element are identical in size, mass and chemical properties *** Atoms of different elements are different from those of other elements Different atoms combine to form compounds Atoms are separated, combined or rearranged in chemical reactions JOHN DALTON ***
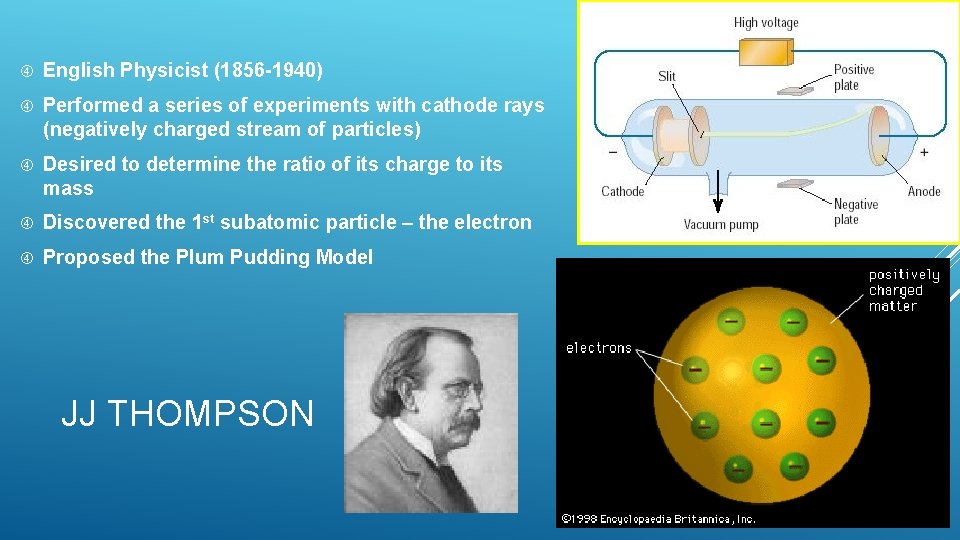
English Physicist (1856 -1940) Performed a series of experiments with cathode rays (negatively charged stream of particles) Desired to determine the ratio of its charge to its mass Discovered the 1 st subatomic particle – the electron Proposed the Plum Pudding Model JJ THOMPSON
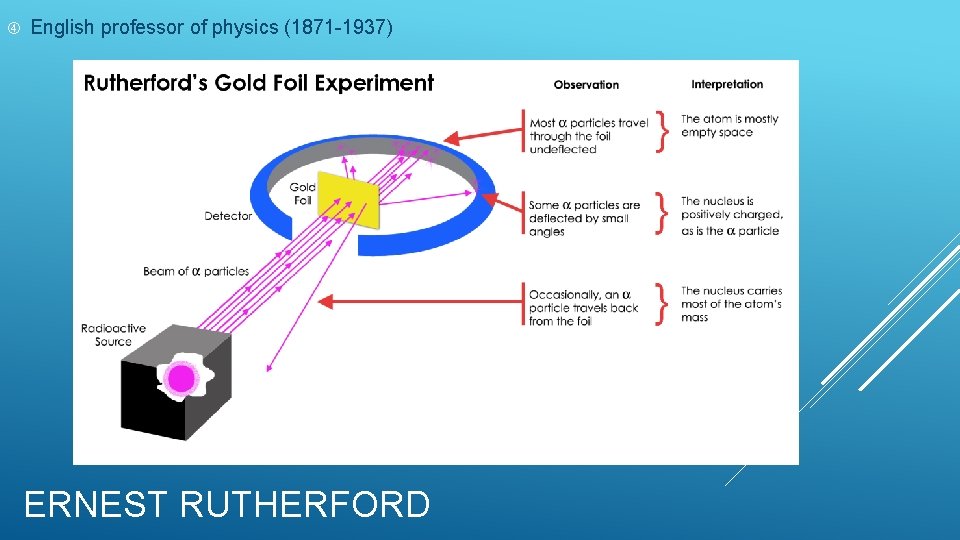
English professor of physics (1871 -1937) ERNEST RUTHERFORD

Expected the alpha particles to go through the gold foil But saw the alpha particles get deflected at large angles Atoms positive charge was located in a small, dense area called the nucleus Positively charged particles were called protons Suggested most of the atom consisted of empty space ERNEST RUTHERFORD

Scientists knew that atoms were neutral which meant that number of protons and number of electrons were equal But those two particles did not account for the mass of the atom – what else was there? HOW TO ACCOUNT FOR THE MISSING MASS?
English Physicist and Rutherford’s co-worker (1891 - 1974) Discovered the nucleus also contained a neutron A subatomic particle that has no charge but the same mass as the proton JAMES CHADWICK
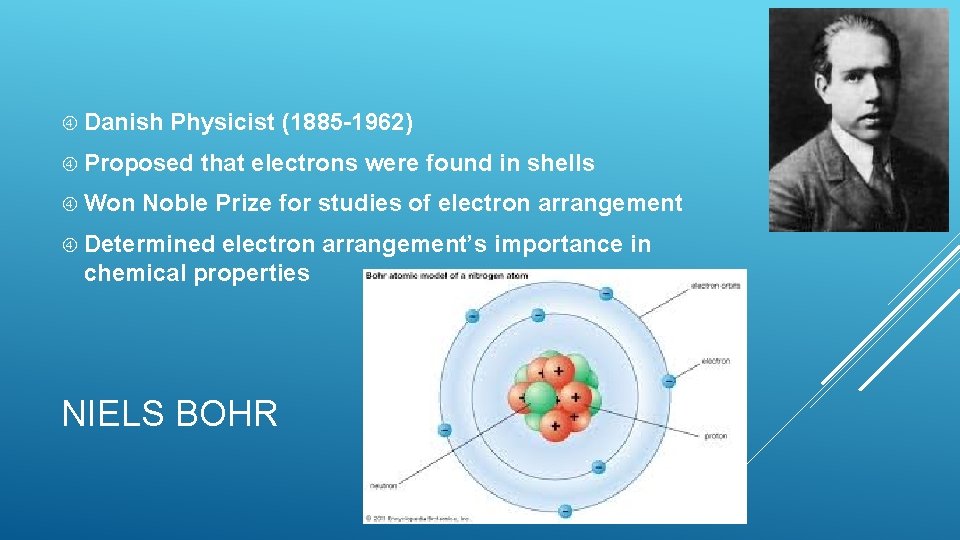
Danish Physicist (1885 -1962) Proposed Won that electrons were found in shells Noble Prize for studies of electron arrangement Determined electron arrangement’s importance in chemical properties NIELS BOHR
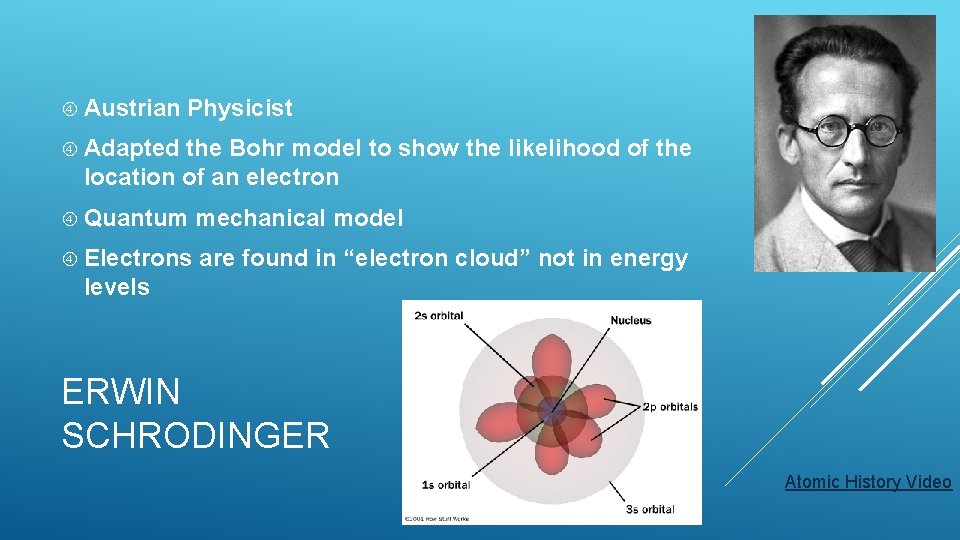
Austrian Physicist Adapted the Bohr model to show the likelihood of the location of an electron Quantum mechanical model Electrons are found in “electron cloud” not in energy levels ERWIN SCHRODINGER Atomic History Video
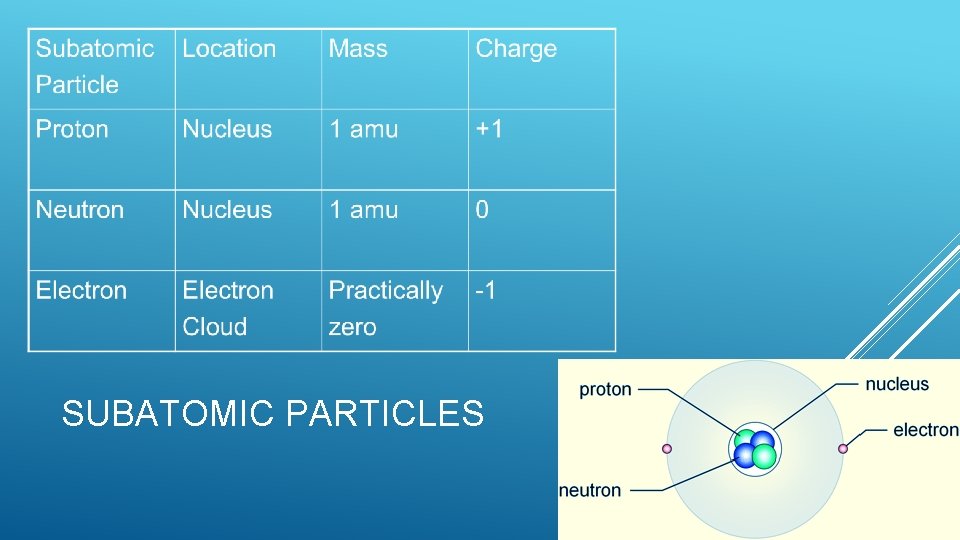
SUBATOMIC PARTICLES
- Slides: 12