HISTORY OF THE ATOM Development of the Atomic
















- Slides: 28

HISTORY OF THE ATOM

Development of the Atomic Model 440 B. C. (Greek Philosopher) Democritus matter is formed of small pieces that could not be cut into smaller parts Atomos- “uncuttable” Remember ancient Greeks did not perform experiments

Dalton’s Atomic Theory - 1803 All elements are composed of atoms that cannot be divided All atoms of the same element are exactly alike and have the same mass. Atoms of different elements are different and have different masses

Dalton’s Atomic Theory cont. An atom of one element cannot be changed into an atom of a different element. Atoms cannot be created or destroyed in any chemical change, only rearranged Every compound is composed of atoms of different elements, combined in a specific ratio

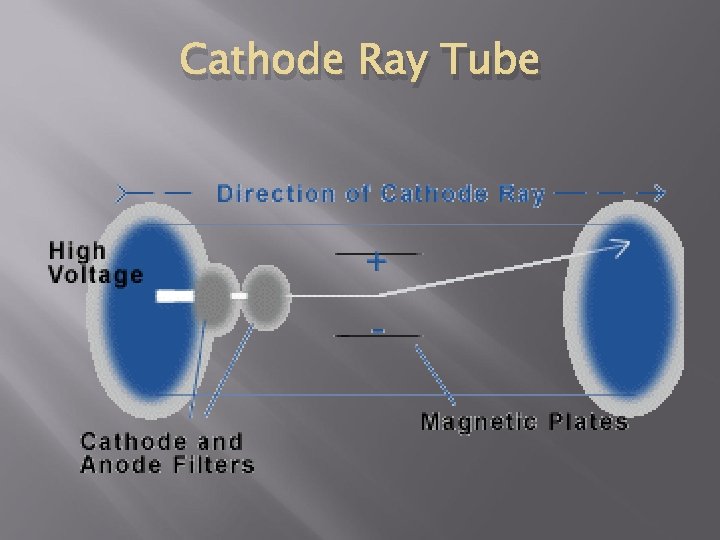
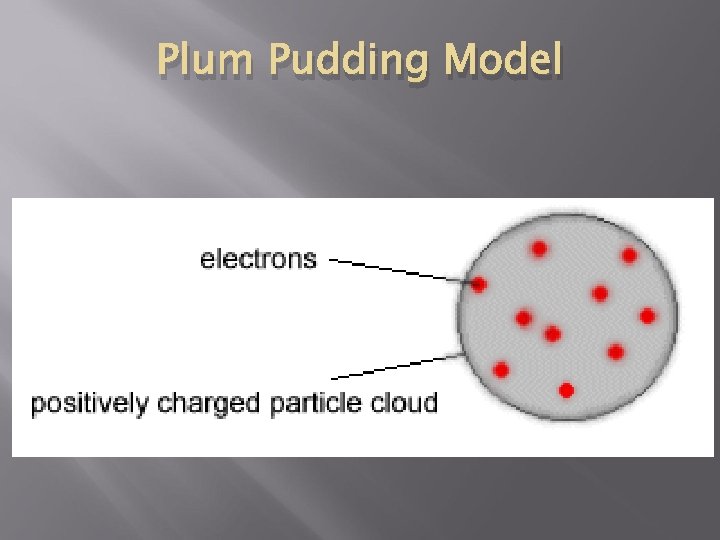
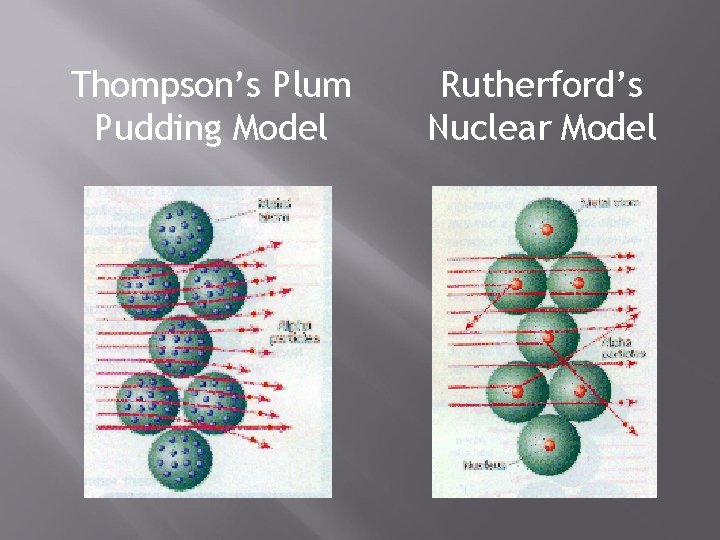
J. J Thomson’s Model - 1897 Used a Cathode Ray Tube that passes an electrical current through a vacuum tube Found that the beam would always bend toward the positive magnet Atom consisted of negative charges scattered throughout a ball of positive charges Become known as electrons Plum Pudding Model

Cathode Ray Tube

Plum Pudding Model

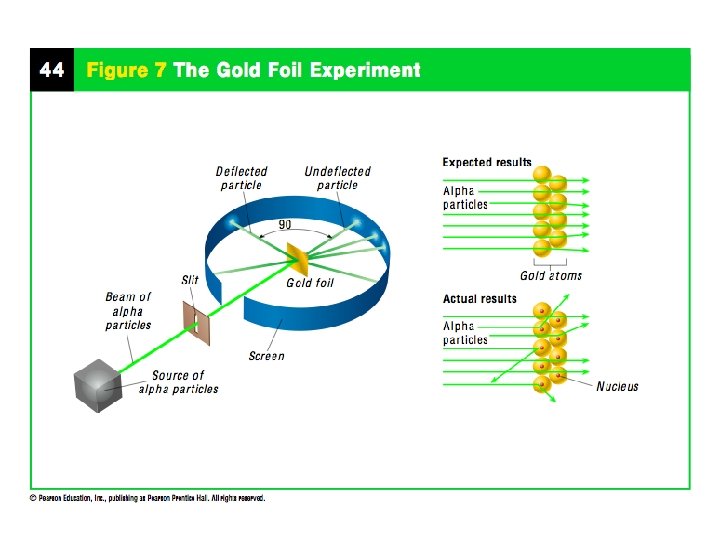
Rutherford’s Model - 1911 Ernest Rutherford was a student of Thomson who found evidence that countered Thomson’s Model Aimed positively charged particles at a thin sheet of gold


Rutherford’s Model cont. Atoms in the gold foil were mostly empty space allowing the particles to pass through but there is a small dense positively charged center of the atom causing the deflections Proposed the Nuclear Model

Thompson’s Plum Pudding Model Rutherford’s Nuclear Model

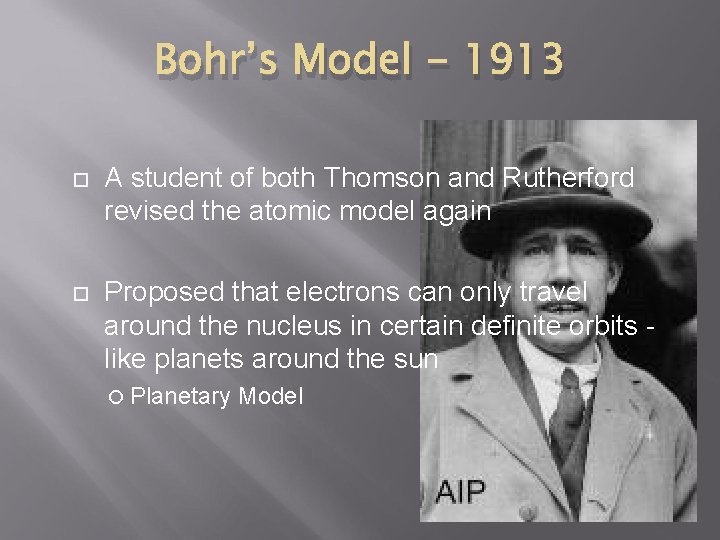
Bohr’s Model - 1913 A student of both Thomson and Rutherford revised the atomic model again Proposed that electrons can only travel around the nucleus in certain definite orbits like planets around the sun Planetary Model

A Cloud of Electrons - 1920’s Schrodinger and Heisenberg Electrons do not orbit, they can be anywhere in a cloudlike region around the nucleus Electron’s movement is related to its energy level - region at a fixed distance from the nucleus These levels affect the way the atom reacts with other atoms Paths electrons travel cannot be determined

Atoms What all elements are composed of All atoms of the same element has the same mass Compounds contain atoms of more than 1 element Always NEUTRAL

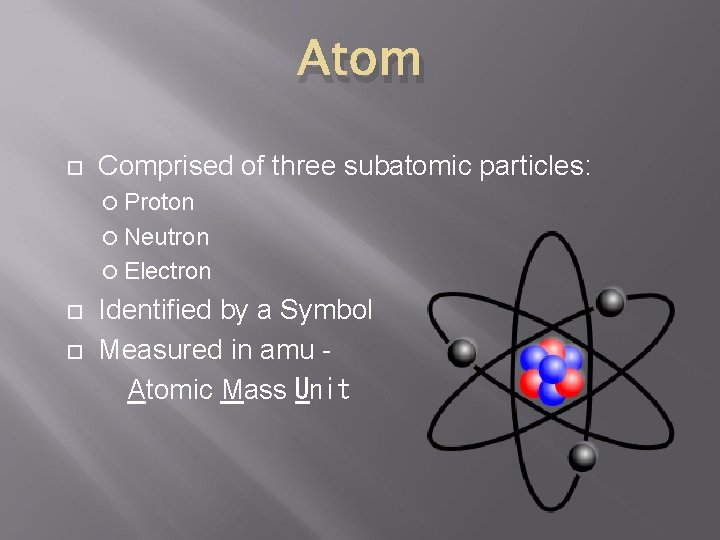
Atom Comprised of three subatomic particles: Proton Neutron Electron Identified by a Symbol Measured in amu Atomic Mass Unit

Proton Has a POSITIVE charge +1 Located inside the nucleus Has a mass of 1 amu

Neutron Has NO charge Located inside the nucleus Has a mass of 1 amu

Electron Has a NEGATIVE charge -1 Located outside the nucleus in the electron cloud Has a mass of 1/1800 amu

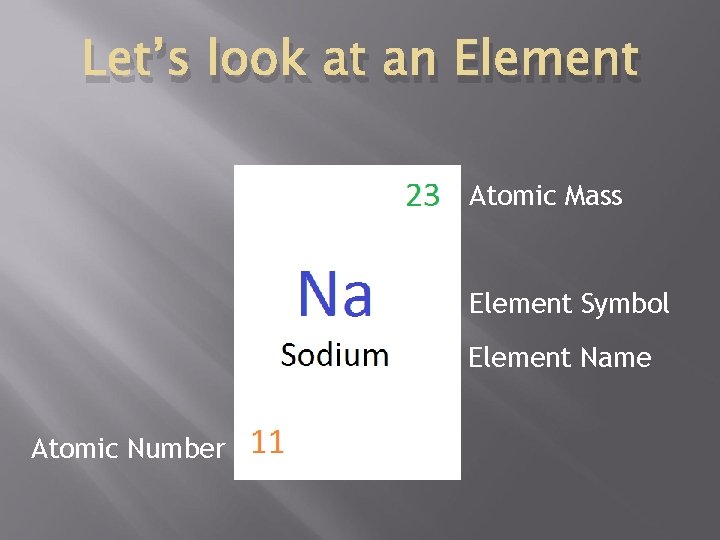
Let’s look at an Element Atomic Mass Element Symbol Element Name Atomic Number

Atomic Number Represented by the letter Z Equals to the number of protons Since positive = negative; protons = electrons Therefore Z = protons = electrons Determines the identity of an element

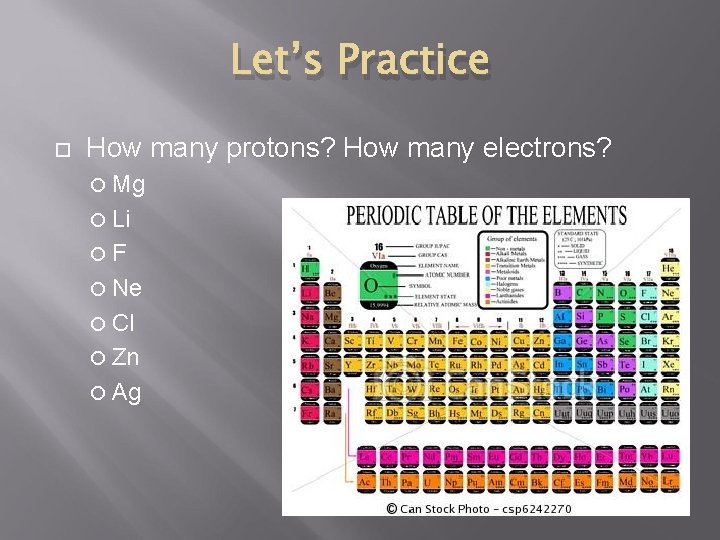
Let’s Practice How many protons? How many electrons? Mg Li F Ne Cl Zn Ag

More Practice What element has 11 protons? Which element has 27 electrons? Which element has 13 electrons?

Atomic Mass is equal to the number of protons + number of neutrons What do you think we don’t include electrons in this number? ?

Isotopes When two atoms of the same element have the same number of protons but DIFFERENT number of neutrons Different atomic mass number

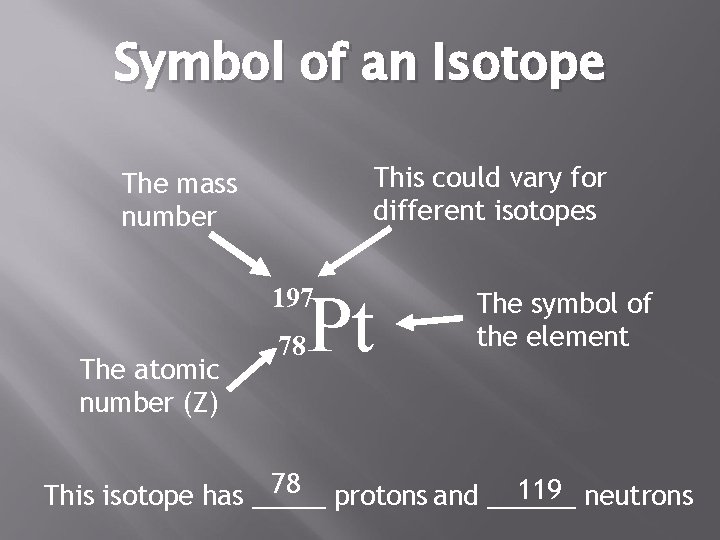
Symbol of an Isotope This could vary for different isotopes The mass number Pt 197 The atomic number (Z) 78 The symbol of the element 78 protons and ______ 119 neutrons This isotope has _____

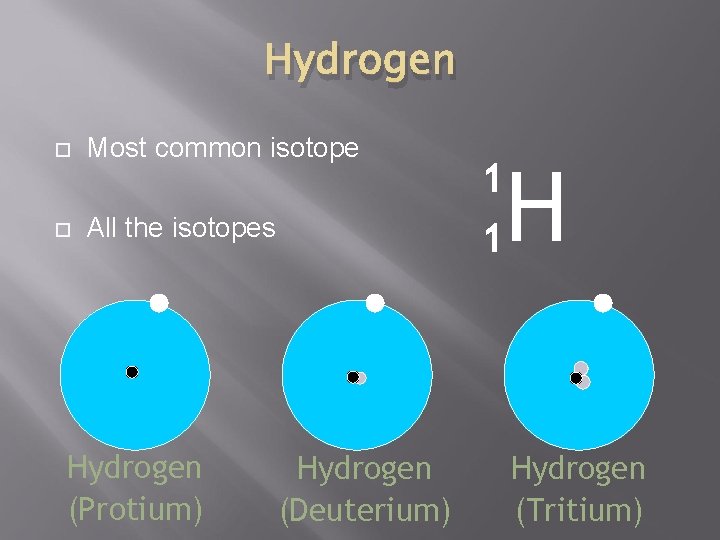
Hydrogen Most common isotope All the isotopes Hydrogen (Protium) Hydrogen (Deuterium) 1 1 H Hydrogen (Tritium)

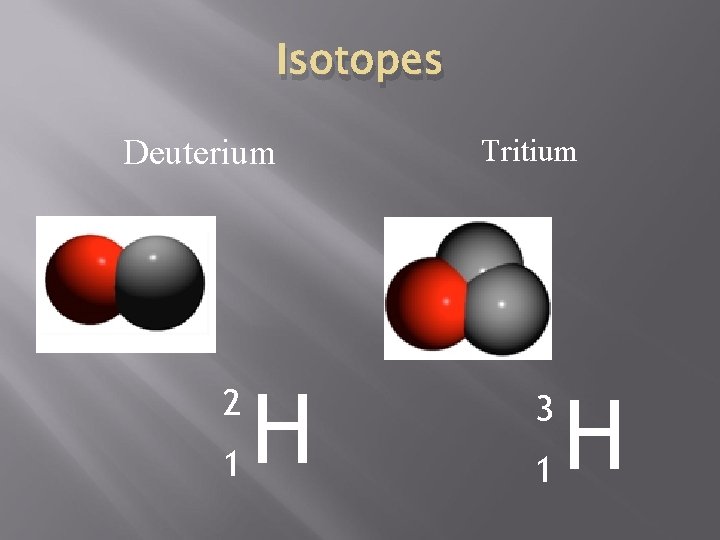
Isotopes Deuterium 2 1 H Tritium 3 1 H

Review Z (atomic number) = p+ = e- Atomic Mass = p+ + n 0 Isotope - different number of neutrons