History of Periodic Table History of Periodic Table

History of Periodic Table

History of Periodic Table n By 1860’s, about 70 elements had been discovered. n No similarities or patterns existed among the elements.

Dimitri Mendeleev (1834 -1907) n Published table in 1869 n Used vertical columns to list by increasing atomic mass, and horizontal rows to arrange by similar traits. n Predicted that new elements would later be discovered that would fit into the “blank” spaces in his table.


Henry Moseley (1887 -1915) n British physicist. n Discovered nuclear charge (protons). n Arranged P. T. by increasing atomic number.

PERIODIC TABLE

Organization of the Periodic Table

The modern periodic table is arranged in order of increasing atomic number. And increasing atomic mass in most cases.

Periodic Law n The physical and chemical properties of the elements are a periodic function of their atomic numbers! n Periodic = repeating
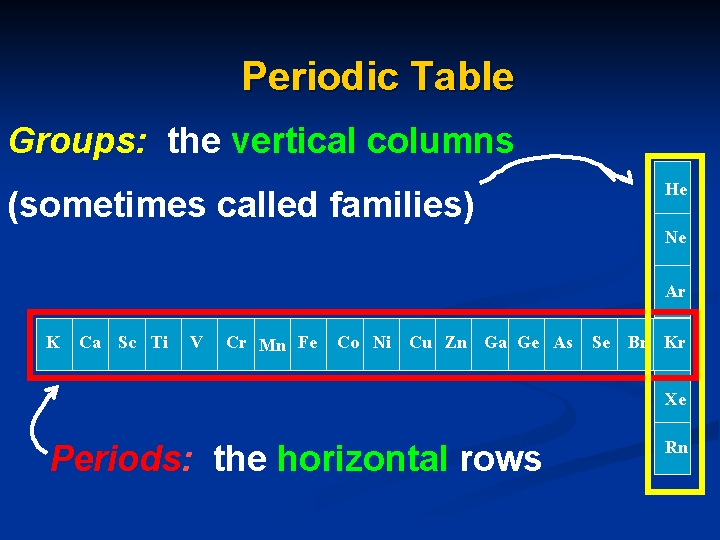
Periodic Table Groups: the vertical columns He (sometimes called families) Ne Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Xe Periods: the horizontal rows Rn
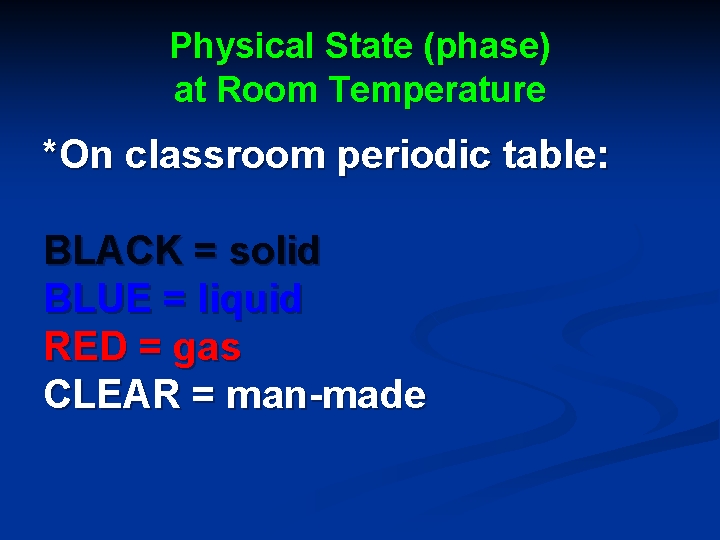
Physical State (phase) at Room Temperature *On classroom periodic table: BLACK = solid BLUE = liquid RED = gas CLEAR = man-made

1 A H Metals, Non-Metals, & Metalloids 2 A Li Be Na Mg K Rb 3 A B 3 B 4 B 5 B 6 B 7 B 8 B Ca Sc Ti Sr Y Cs Ba 8 A Zr La Hf Fr Ra Ac Rf V Cr Mn Fe 8 B 8 B 1 B 2 B Co Ni 4 A 5 A 6 A 7 A He C N O F Ne Al Si P S Cl Ar Se Br Kr Cu Zn Ga Ge As Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te Ta W Re Os Db Sg Bh Hs Ir Pt Au Hg Tl Pb Bi I Xe Po At Rn Mt Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr

Metals n Left side of the “stairstep” on periodic table n except H n Properties: n lustrous (shiny) n good conductors of heat & electricity n malleable & ductile n solids at RT (except Hg)

Non-Metals n Right side of “stairstep” n plus Hydrogen n Properties: n Dull appearance n Brittle when solids n Do not conduct heat or electricity well n May be solid, liquid or gas at RT

Metalloids (Semi-metals) n Touch “stairstep” on at least one full side. n Exception: Aluminum n Properties of both metals and non-metals

1 A H Metals, Non-Metals, & Metalloids 2 A Li Be Na Mg K Rb 3 A B 3 B 4 B 5 B 6 B 7 B 8 B Ca Sc Ti Sr Y Cs Ba 8 A Zr La Hf Fr Ra Ac Rf V Cr Mn Fe 8 B 8 B 1 B 2 B Co Ni 4 A 5 A 6 A 7 A He C N O F Ne Al Si P S Cl Ar Se Br Kr Cu Zn Ga Ge As Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te Ta W Re Os Db Sg Bh Hs Ir Pt Au Hg Tl Pb Bi I Xe Po At Rn Mt Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr

1 H Representative Elements 2 s & p blocks Li Be Na Mg K Rb 4 5 6 7 8 9 Ca Sc Ti V Cr Mn Fe Co Ni 3 Sr Y Cs Ba Zr La Hf Fr Ra Ac Rf 10 11 12 18 13 14 15 16 17 He B C N O F Ne Al Si P S Cl Ar Se Br Kr Cu Zn Ga Ge As Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Ta W Po At Rn Re Os Db Sg Bh Hs Ir Pt Au Hg Tl Pb Bi Xe Mt Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr

1 H 2 Li Be Na Mg K Rb 13 14 15 16 17 B d block 4 5 6 7 Ca Sc Ti V Cr Mn Fe 3 Sr Y Cs Ba 18 Transition Elements Zr La Hf Fr Ra Ac Rf 8 9 10 11 12 Co Ni He C N O F Ne Al Si P S Cl Ar Se Br Kr Cu Zn Ga Ge As Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Ta W Po At Rn Re Os Db Sg Bh Hs Ir Pt Au Hg Tl Pb Bi Xe Mt Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr

1 H Inner Transition (Lanthanides) 2 Li Be Na Mg K Rb 3 7 8 5 6 Ca Sc Ti V Cr Mn Fe Sr Y Zr La Hf Fr Ra Ac Rf 14 15 16 17 He C N O F Ne Al Si P S Cl Ar Se Br Kr 13 B f block 4 Cs Ba 18 9 10 11 12 Co Ni Cu Zn Ga Ge As Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Ta W Po At Rn Re Os Db Sg Bh Hs Ir Pt Au Hg Tl Pb Bi Xe Mt Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr

f block 1 H 2 Inner Transition Li Be Na Mg K Rb 3 7 8 5 6 Ca Sc Ti V Cr Mn Fe Sr Y Zr La Hf Fr Ra Ac Rf 9 10 11 12 Co Ni 14 15 16 17 He C N O F Ne Al Si P S Cl Ar Se Br Kr 13 B (Actinides) 4 Cs Ba 18 Cu Zn Ga Ge As Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Ta W Po At Rn Re Os Db Sg Bh Hs Ir Pt Au Hg Tl Pb Bi Xe Mt Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr

1 H 18 Alkali Metals 2 13 14 15 16 17 B Li Be Na Mg K Rb 3 7 8 4 5 6 Ca Sc Ti V Cr Mn Fe Sr Y Cs Ba Zr La Hf Fr Ra Ac Rf 9 10 11 12 Co Ni He C N O F Ne Al Si P S Cl Ar Se Br Kr Cu Zn Ga Ge As Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Ta W Po At Rn Re Os Db Sg Bh Hs Ir Pt Au Hg Tl Pb Bi Xe Mt Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr

1 H 18 Alkaline Earth Metals 2 Li Be Na Mg K Rb He C N O F Ne Al Si P S Cl Ar Se Br Kr B 3 7 8 4 5 6 Ca Sc Ti V Cr Mn Fe Sr Y Cs Ba 14 15 16 17 13 Zr La Hf Fr Ra Ac Rf 9 10 11 12 Co Ni Cu Zn Ga Ge As Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Ta W Po At Rn Re Os Db Sg Bh Hs Ir Pt Au Hg Tl Pb Bi Xe Mt Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr

1 A H 8 A Halogens 2 A 3 A Li Be Na Mg K Rb B 3 B 4 B 5 B 6 B 7 B 8 B Ca Sc Ti Sr Y Cs Ba Zr La Hf Fr Ra Ac Rf V Cr Mn Fe 8 B 8 B 1 B 2 B Co Ni 4 A 5 A 6 A 7 A He C N O F Ne Al Si P S Cl Ar Se Br Kr Cu Zn Ga Ge As Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Ta W Po At Rn Re Os Db Sg Bh Hs Ir Pt Au Hg Tl Pb Bi Xe Mt Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr

1 A H 8 A Noble Gases 2 A 3 A Li Be Na Mg K Rb B 3 B 4 B 5 B 6 B 7 B 8 B Ca Sc Ti Sr Y Cs Ba Zr La Hf Fr Ra Ac Rf V Cr Mn Fe 8 B 8 B 1 B 2 B Co Ni 4 A 5 A 6 A 7 A He C N O F Ne Al Si P S Cl Ar Se Br Kr Cu Zn Ga Ge As Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Ta W Po At Rn Re Os Db Sg Bh Hs Ir Pt Au Hg Tl Pb Bi Xe Mt Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr

PERIODIC TRENDS

n 3 Factors affect trends: 1)Nuclear charge: “Pull” on valence e- by p+ in nucleus 2)Shielding effect: Interference by core e- on p+ “pull” on valence e-. 3)Electron repulsion: valence e- repel

Why do the trends occur? n. As you move ACROSS a period, valence electrons are added to the same P. E. L.

n Nuclear charge increases because you are adding more e- (and p+), but at the same distance from nucleus, so p+ “pull” on valence e- is greater.

n Shielding not affected (no increase in the number of core e- to get in the way)

n Electron repulsion increases because you are adding more electrons.

Why do the trends occur? n. As you move DOWN a group, valence electrons are added to different P. E. L.

n Effective Nuclear charge decreases because you are adding more e- (and p+), but they are further from the nucleus, so the “pull” on valence e- by p+ is less effective.

n Shielding increases because as e- are added at P. E. L. further from the nucleus, there are more core e- to interfere.

n. Electron repulsion increases because you are adding more electrons.

Atomic Radii (size of atom) Distance from the center of an atom's nucleus to the edge of its electron cloud. (remember the edge of an electron cloud is undefined. ) atom

Periodic Table Decreases Increases Periodic Trend-Atomic Size

Dr. Lanzaflame; Atomic radii; www. monroecc. edu/wusers/flanzafame/Per. Radii. pdf

Ionic Size (size of ion) n Ion - an atom that has lost or gained electron(s). n Ionic Radius: Distance from the center of an ion’s nucleus to its outermost electron.

n. Positive ions (cations) are smaller than their neutral atoms n. Negative ions (anions) are larger than their neutral atoms

Black = atom red = + ion blue = -ion

Periodic Table Decreases Increases Periodic Trend-Ionic Size

Ionization Energy The amount of energy needed to remove one valence electron from a gaseous atom of an element.

Periodic Table Increases Decreases Periodic Trend-Ionization Energy

Electronegativity The tendency of an atom to attract electrons to itself in a chemical bond.

Periodic Table Increases Decreases Periodic Trend-Electronegativity

Metallic Character n Properties of metals: nlustrous (shiny) ngood conductors of heat & electricity nmalleable & ductile nsolids at RT (except Hg)

Periodic Table Decreases Increases Periodic Trend-Metallic Character

Electron Affinity n. The energy change involved when a gaseous atom of an element gains an electron. n. Cl (g) + e ---> Cl (g)

Periodic Table increases decrease Periodic Trend-Electron Affinity

Periodic Properties of Elements n Chemical and physical properties of the elements vary with their position in the periodic table. n Atomic size n Size of Ion n Ionization energy n Electron affinity n Metallic character n Electronegativity

I. Atomic Size Atomic radius: Distance from the center of an atom's nucleus to the edge of its electron cloud. (remember the edge of an electron cloud is undefined. ) atom

Trend in Atomic Size n Within a period, atoms generally get smaller as you move from left to right. n WHY? Because of the increasing positive charge of the nucleus.

Trend In Atomic Size n Within a group, atoms generally get larger as you move from top to bottom. n WHY? Electrons added require additional energy levels which are further from the nucleus.

Dr. Lanzaflame; Atomic radii; www. monroecc. edu/wusers/flanzafame/Per. Radii. pdf

Periodic Table Increases Periodic Trend-Atomic Size Increases Lower “lefter” larger

Periodic Properties--Atomic Size n Example: Which element would have the larger atomic radius, Ar or Br? Br should have the larger radius n more towards the bottom n more towards the left side

II. Ionic Size n Ion - an atom that has lost or gained electron(s). n Ionic Radius: Distance from the center of an ion’s nucleus to its outermost electron.

n Positive ions (cations) are smaller than their neutral atoms n Negative ions (anions) are larger than their neutral atoms

Black = atom red = + ion blue = -ion

WHY? n When a cation is formed, it has usually lost one entire layer of its electron cloud. n Also, the nuclear charge has a greater effect on the remaining electrons.

What about anions? n When an anion is formed, the extra electron(s) add to the repulsion between the electrons. n Also, the nuclear charge has a less of an effect on the increased number of electrons.

Trend in Ionic Size n Within a period, ions tend to become smaller as you move from left to right. n Within a group, ions tend to become larger as you move from top to bottom.

Periodic Table Increases Periodic Trend-Ionic Size Increases Lower “lefter” larger

III. Ionization Energy Ionization energy: The amount of energy needed to remove one valence electron from a gaseous atom of an element.

Trend in Ionization Energy n Within a period, ionization energy tends to increase as you move from left to right. n Within a group, ionization energy tends to decrease as you move from top to bottom.

Periodic Table Decreases Periodic Trend-Ionization Energy

IV. Electron Affinity n The energy needed for a gaseous atom of an element to gain an electron.

Trend in Electron Affinity n Within a period, electron affinity tends to increases across the chart. n Within a group, electron affinity tends to decreases down the chart.

Periodic Table decrease Periodic Trend-Electron Affinity decreases Lower “lefter” larger

V. Electronegativity The tendency of an atom to attract electrons to itself in a chemical bond.

Trend in Electronegativity n Within a period, electronegativity tends to increase as you move from left to right. n Within a group, electronegativity tends to decrease as you move from top to bottom.

Periodic Table Decreases Periodic Trend-Electronegativity

n. Fluorine is the most electronegative of all! n. Atoms gain, lose or share electrons in an attempt to complete an octet in the outer most energy level.

Periodicity of Chemical and Physical Properties 1 2 H He Li Be Inert gas 3 4 Soft reactive metal 9 10 11 12 17 18 19 20 F Ne Na Mg Cl Ar K Inert gas Soft reactive metal Inert gas Ca Soft reactive metal
- Slides: 74