History of Opium http opioids comtimelineindex html Constituents
































- Slides: 37

History of Opium http: //opioids. com/timeline/index. html

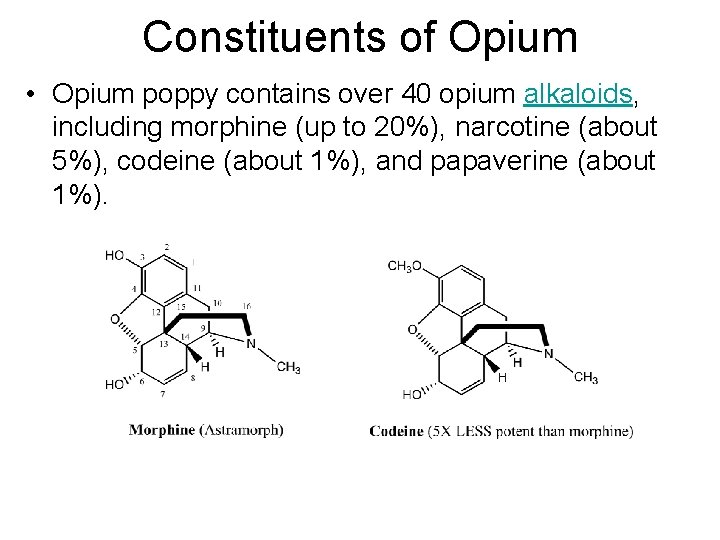
Constituents of Opium • Opium poppy contains over 40 opium alkaloids, including morphine (up to 20%), narcotine (about 5%), codeine (about 1%), and papaverine (about 1%).

The History of Morphine • http: //opioids. com/morphine/200 anniv. html

History of Morphine • Doctors had long hunted for effective ways to administer drugs without ingesting them. Taken orally, opium is liable to cause unpleasant gastric sideeffects. The development of the hypodermic syringe in the midnineteenth century allowed the injection of pure morphine. Both in Europe and America, members of high society and middle-class professionals alike would jack up daily; poor folk couldn't afford to inject drugs.

The History of Morphine • Morphinism became rampant in the USA after its extensive use by injured soldiers on both sides of the Civil War. In late nineteenth-century America, opiates were cheap, legal and abundant. In the judgement of one historian, America became "a dope fiend's paradise". Moreover it was believed that injecting morphine wasn't addictive. Quitting habitual opium use can cause malaise, flu-like symptoms, and depression; morphine seemed an excellent cure. In China, for instance, early twentieth century missionaries handed out antiopium remedies in such profusion that the pills became known as "Jesus Opium"; their active ingredient was morphine.

Side effects of morphine • Morphine has many side effects. The most dangerous is respiratory depression. Minor degrees of respiratory depression may be detected following standard doses of morphine, but this is not clinically important. With higher doses or in frail patients, the respiratory rate decreases, the patient becomes increasingly sedated, and the pupils very small. Common side effects are nausea and vomiting due to a central action of morphine stimulating one of the centres in the brain concerned with vomiting called the chemotactic trigger zone. Other central nervous system side effects of morphine are cough suppression, sedation, and dependence leading to addiction.

Side effects of morphine • Morphine also has an effect on the muscle of the bowel and urinary tract, causing the sphincter to contract and reduce the peristalsis (the wavelike movements of the bowel muscle that propel its contents forwards). This results in a delayed emptying of the stomach, constipation, and may also lead to urinary retention.

Structure of Morphine

‘Tinkering’ with the structure of morphine produced heroin

From aspirin to heroin? • The father of modern medicine was Hippocrates, who lived sometime between 460 B. C and 377 B. C. Hippocrates left historical records of pain relief treatments, including the use of powder made from the bark and leaves of the willow tree to help heal headaches, pains and fevers. • By 1829, scientists discovered that it was the compound called salicin in willow plants which gave you the pain relief.

From aspirin to heroin? • According to "From A Miracle Drug" written by Sophie Jourdier for the Royal Society of Chemistry: "It was not long before the active ingredient in willow bark was isolated; in 1828, Johann Buchner, professor of pharmacy at the University of Munich, isolated a tiny amount of bitter tasting yellow, needle-like crystals, which he called salicin.

From aspirin to heroin? • Two Italians, Brugnatelli and Fontana, had in fact already obtained salicin in 1826, but in a highly impure form. By 1829, [French chemist] Henri Leroux had improved the extraction procedure to obtain about 30 g from 1. 5 kg of bark. In 1838, Raffaele Piria [an Italian chemist] then working at the Sorbonne in Paris, split salicin into a sugar and an aromatic component (salicylaldehyde) and converted the latter, by hydrolysis and oxidation, to an acid of crystallised colourless needles, which he named salicylic acid. "

From aspirin to heroin? • The problem was that salicylic acid was tough on stomachs and a means of 'buffering' the compound was searched for. The first person to do so was a French chemist named Charles Frederic Gerhardt. In 1853, Gerhardt neutralized salicylic acid by buffering it with sodium (sodium salicylate) and acetyl chloride, creating acetylsalicylic acid. Gerhardt's product worked but he had no desire to market it and abandoned his discovery.

From aspirin to heroin? • In 1899, a German chemist named Felix Hoffmann, who worked for a German company called Bayer, rediscovered Gerhardt's formula. Felix Hoffmann made some of the formula and gave it to his father who was suffering from the pain of arthritis. With good results, Felix Hoffmann then convinced Bayer to market the new wonder drug. Aspirin was patented on March 6, 1889.

From aspirin to heroin? • The folks at Bayer came up with the name Aspirin, it comes from the 'A" in acetyl chloride, the "spir" in spiraea ulmaria (the plant they derived the salicylic acid from) and the 'in' was a then familiar name ending for medicines.

From aspirin to heroin? O-Acetylsalicylic acid is a better pain reliever than Salicylic acid. It is also less irritating to the stomach.

From aspirin to heroin? • The following is taken from: • http: //opioids. com/heroinhistory. h tml • Source: Sunday Times. Date: 13 September 1998

From aspirin to heroin? • Heinrich Dreser, chemist and opportunist, was one of the most influential men of his age. • Born in 1860, in Darmstadt, the son of a physics professor, he showed promise as a chemist from an early age. After receiving his doctorate from Heidelberg University, he worked in various laboratories before becoming a professor at Bonn University in 1893. Four years later he joined the Bayer Company, where he was in charge of testing the efficacy and safety of new drugs.

From aspirin to heroin? • Dreser was admired for his thorough, methodical approach, and for his innovations in testing (he was, for example, the first chemist to use animal experiments on an industrial scale). The credit for originating new products for Bayer belonged, strictly speaking, to the researcher Arthur Eichengruen, but Dreser had the power to decide which new products would be developed. He had also negotiated a special deal which guaranteed him a share of the profits from products he launched.

From aspirin to heroin? • In 1897 the Bayer chemist Felix Hoffmann, acting on Eichengruen's instructions, discovered a new process for modifying salicyclic acid (a remedy for fever and inflammation which unfortunately has excruciating digestive side effects) to produce acetylsalicyclic acid (ASA).

From aspirin to heroin? • Eichengruen enthusiastically recommended ASA to Dreser in 1898. Dreser, after cursory consideration, rejected it. Ostensibly, his objection was that ASA would have an "enfeebling" action on the heart. "The product has no value, " he pronounced confidently. But the real problem was almost certainly that he had another product on his mind whose impending success he was anxious not to jeopardise. This was heroin.

From aspirin to heroin? • The drug that Bayer launched under the trademark Heroin in 1898 was not an original discovery. Diacetylmorphine, a white, odourless, bitter, crystalline powder deriving from morphine, had been invented in 1874 by an English chemist, C R Wright.

From aspirin to heroin? • Diacetylmorphine was first synthesised in the Bayer laboratory in 1897 - by Hoffmann, two weeks after he first synthesised ASA. The work seems to have been initiated by Dreser, who was by then aware of Wright's discovery, even though he subsequently implied that heroin was an original Bayer invention.

From aspirin to heroin? • In November 1898, Dreser presented the drug to the Congress of German Naturalists and Physicians, claiming it was 10 times more effective as a cough medicine than codeine, but had only a tenth of its toxic effects. It was also more effective than morphine as a painkiller. It was safe. It wasn't habit-forming. In short, it was a wonder drug - the Viagra of its day. • "What we don't recognise now, " says David Muso, professor of psychiatry and the history of medicine at Yale Medical School, "is that this met what was then a desperate need - not for a painkiller, but for a cough remedy". • Tuberculosis and pneumonia were then the leading causes of death, and even routine coughs and colds could be severely incapacitating. Heroin, which both depresses respiration and, as a sedative, gives a restorative night's sleep, seemed a godsend.

From aspirin to heroin? • By 1899, Bayer was producing about a ton of heroin a year, and exporting the drug to 23 countries. The country where it really took off was the US, where there was already a large population of morphine addicts, a craze for patent medicines, and a relatively lax regulatory framework. Manufacturers of cough syrup were soon lacing their products with Bayer heroin. • There were heroin pastilles, heroin cough lozenges, heroin tablets, water-soluble heroin salts and a heroin elixir in a glycerine solution. Bayer never advertised heroin to the public but the publicity material it sent to physicians was unambiguous. One flyer described the product thus: "Heroin: the Sedative for Coughs. . . order a supply from your jobber. ”

From aspirin to heroin? • But worrying rumours were surfacing. As early as 1899, researchers began to report patients developing "tolerance" to the drug, while a German researcher denounced it as "an extremely dangerous poison". By 1902 - when heroin sales were accounting for roughly five percent of Bayer's net profits - French and American researchers were reporting cases of "heroinism" and addiction. • Had heroin been his only pet project, this disappointment could have spelt career disaster.

From aspirin to heroin (and back)! • Luckily, although his first "baby" was showing signs of turning into a monster, Dreser had belatedly adopted another: aspirin. Eichengruen, refusing to accept Dreser's rejection of ASA, had continued to investigate it and to lobby for its development. Eventually, Dreser recognised which way the wind was blowing, tested ASA on himself (as well as on his laboratory of rabbits), and finally published an enthusiastic scientific paper recommending it, particularly for the treatment of rheumatism - but omitting to mention the contributions of Eichengruen and Hoffmann. In February 1899, the brand name "Aspirin" was registered, and in June, Dreser presided over its launch.

And Back! • Like heroin, aspirin more or less sold itself. As a painkiller without undesirable side effects, it was - and remained for decades unique. By the end of 1899 it was being used all over Europe and the US, and by the time the heroin bubble burst, aspirin had more than filled the gap. Bayer was on its way to becoming an industrial giant. Hoffman and Eichengruen do not seem to have received any special compensation for their efforts. For Dreser, though, the rewards were spectacular.

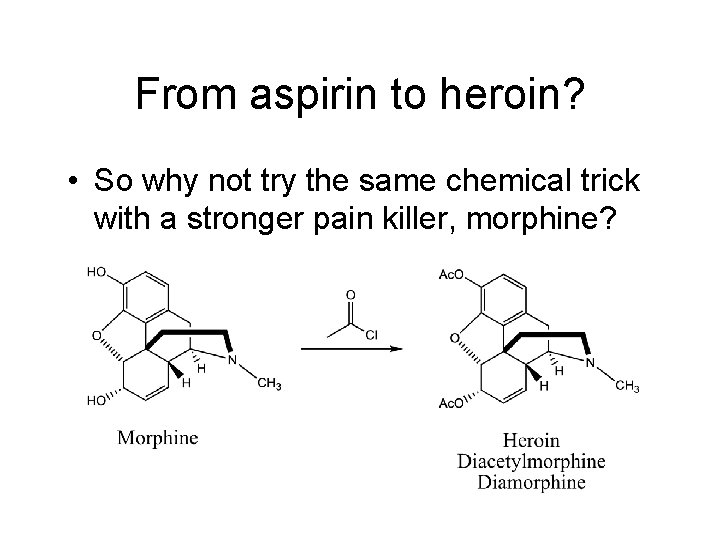
From aspirin to heroin? • So why not try the same chemical trick with a stronger pain killer, morphine?

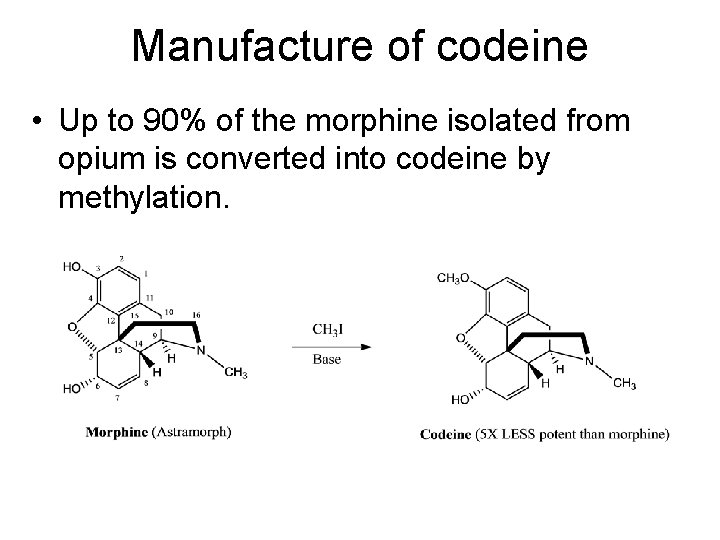
Manufacture of codeine • Up to 90% of the morphine isolated from opium is converted into codeine by methylation.

Codeine is demethylated back to morphine in the liver • To experience the painkilling properties of codeine the body must first convert it into morphine. Codeine is readily absorbed by the gastrointestinal tract, becoming quickly transported to various tissues throughout the body. Codeine does not accumulate in body tissues because it is metabolised by the liver and its metabolic products are excreted by the kidneys. The process by which codeine is metabolised is known as glucuronidation. Through Odemethylation the codeine is converted into morphine and through N-demethylation it becomes norcodeine. The metabolism rate is approximately 30 mg of codeine in an hour and about 90% of the drug will be excreted from the body within a day. In most people, only about 10% of codeine is transformed into morphine.

The C 3 hydroxyl group is necessary for activity. (The methyl ether is only 0. 1% as active)

Codeine is a useful cough suppresant • The antitussive and analgesic attributes of codeine also enable it to work as a cough suppressant, especially with dry, nonproductive coughs. It does this by inhibiting the receptor in the cough centre of the medulla oblongata and acting on the brain to reduce the cough reflex, without the suppression of the respiratory centre. Codeine increases the viscosity of bronchial secretions and has a drying effect on the respiratory tract.

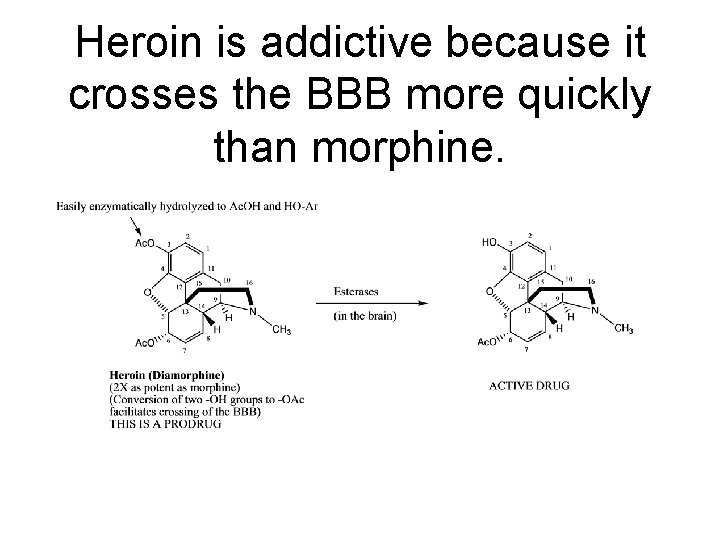
Heroin is addictive because it crosses the BBB more quickly than morphine.

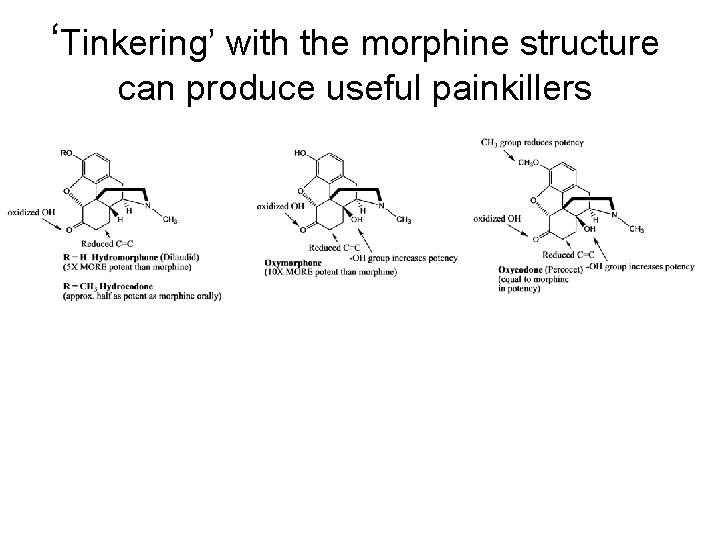
‘Tinkering’ with the morphine structure can produce useful painkillers

‘Tinkering’ with the nitrogen substituent can produce opioid antagonists • Opioid antagonists are used to treat heroin and morphine overdose

These drugs may also be used to treat recovering addicts • Naltrexone is sometimes used for rapid detoxification ("rapid detox") regimens for opioid dependence. The principle of rapid detoxification is to induce opioidreceptor blockade while the patient is in a state of impaired consciousness so as to attenuate the withdrawal symptoms experienced by the patient. Rapid detoxification under general anaesthesia involves an unconscious patient and requires intubation and external ventilation. Rapid detoxification is also possible under sedation. The rapid detoxification procedure is followed by oral naltrexone daily for up to 12 months for opioid dependence management. There a number of practitioners who will use a naltrexone implant placed in the lower abdomen, and more rarely, in the posterior to replace the oral naltrexone. This implant procedure has not been shown scientifically to be successful in "curing" the subject of their addiction, though it does provide a better solution than oral naltrexone for medication compliance reasons. Naltrexone implants are made by at least three companies, though none are FDA approved. There is currently scientific disagreement as to whether this procedure should be performed under local or general anesthesia, due to the rapid, and sometimes severe, withdrawal that occurs from the naltrexone displacing the opiates from the receptor sites.
 Opioids for neuropathic pain
Opioids for neuropathic pain Opium wars defintion
Opium wars defintion Poudre d'opium
Poudre d'opium Opium wars and boxer rebellion
Opium wars and boxer rebellion Opium wars defintion
Opium wars defintion Why did britain begin exporting opium to china?
Why did britain begin exporting opium to china? China opium war political cartoon
China opium war political cartoon Ephedrae herba
Ephedrae herba Opium adalah
Opium adalah Opium chapter 24
Opium chapter 24 Reformatory function of financial system
Reformatory function of financial system Sentence constituents exercises
Sentence constituents exercises Components of soil diagram
Components of soil diagram What are the basic constituents of matter
What are the basic constituents of matter What are the three constituents of the indian parliament
What are the three constituents of the indian parliament How agents, constituents and audiences change negotiations?
How agents, constituents and audiences change negotiations? Csf constituents
Csf constituents What are constituents?
What are constituents? Juxtaglomerular apparatus
Juxtaglomerular apparatus Countercurrent multiplier
Countercurrent multiplier Abnormal constituents of urine
Abnormal constituents of urine Urinalysis result interpretation chart
Urinalysis result interpretation chart Exercise 44
Exercise 44 Epra index constituents
Epra index constituents Canvas доска
Canvas доска Html
Html Doctype html html head
Doctype html html head Bhtml?title=
Bhtml?title= 1
1 Http://geosevillano.blogspot.com/p/blog-page_6.html
Http://geosevillano.blogspot.com/p/blog-page_6.html Davidmlane com hyperstat index html
Davidmlane com hyperstat index html Http://metrocosm.com/global-migration-map.html
Http://metrocosm.com/global-migration-map.html Number of neutrons xenon
Number of neutrons xenon Http://www.kcvs.ca/site/projects/physics.html
Http://www.kcvs.ca/site/projects/physics.html Food chain energy flow
Food chain energy flow Http index.html
Http index.html Http://weather.uwyo.edu/upperair/sounding.html
Http://weather.uwyo.edu/upperair/sounding.html Http://weather.uwyo.edu/upperair/sounding.html
Http://weather.uwyo.edu/upperair/sounding.html