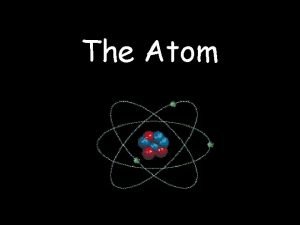
History of Chemistry Early models of the atom











![Average atomic mass [Worksheet] • Based on abundance of isotopes 1)Change % to decimal Average atomic mass [Worksheet] • Based on abundance of isotopes 1)Change % to decimal](https://slidetodoc.com/presentation_image_h/265ce1b76b2fa1010934e28daa8bdc65/image-44.jpg)















![Electron Configuration • N [He]2 s 22 p 3 Electron Configuration • N [He]2 s 22 p 3](https://slidetodoc.com/presentation_image_h/265ce1b76b2fa1010934e28daa8bdc65/image-114.jpg)
![Electron Configuration • Si [Ne]3 s 23 p 2 Electron Configuration • Si [Ne]3 s 23 p 2](https://slidetodoc.com/presentation_image_h/265ce1b76b2fa1010934e28daa8bdc65/image-115.jpg)
![Electron Configuration • Mg [Ne]3 s 2 Electron Configuration • Mg [Ne]3 s 2](https://slidetodoc.com/presentation_image_h/265ce1b76b2fa1010934e28daa8bdc65/image-116.jpg)
![Electron Configuration • Ga [Ar]4 s 23 d 104 p 1 Electron Configuration • Ga [Ar]4 s 23 d 104 p 1](https://slidetodoc.com/presentation_image_h/265ce1b76b2fa1010934e28daa8bdc65/image-117.jpg)
![Periodic Patterns • Example - Germanium [Ar] 2 4 s 10 3 d Periodic Patterns • Example - Germanium [Ar] 2 4 s 10 3 d](https://slidetodoc.com/presentation_image_h/265ce1b76b2fa1010934e28daa8bdc65/image-118.jpg)
![Stability • Electron Configuration Exceptions – Copper EXPECT: ACTUALLY: [Ar] 4 s 2 3 Stability • Electron Configuration Exceptions – Copper EXPECT: ACTUALLY: [Ar] 4 s 2 3](https://slidetodoc.com/presentation_image_h/265ce1b76b2fa1010934e28daa8bdc65/image-121.jpg)
![D. Stability • Electron Configuration Exceptions – Chromium EXPECT: ACTUALLY: [Ar] 4 s 2 D. Stability • Electron Configuration Exceptions – Chromium EXPECT: ACTUALLY: [Ar] 4 s 2](https://slidetodoc.com/presentation_image_h/265ce1b76b2fa1010934e28daa8bdc65/image-124.jpg)
- Slides: 128

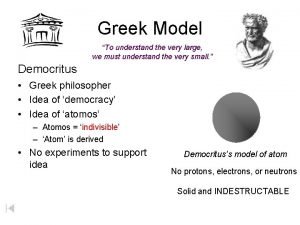
History of Chemistry Early models of the atom • Democritus- early Greek philosopher • matter made up of tiny indivisible particles called atoms •

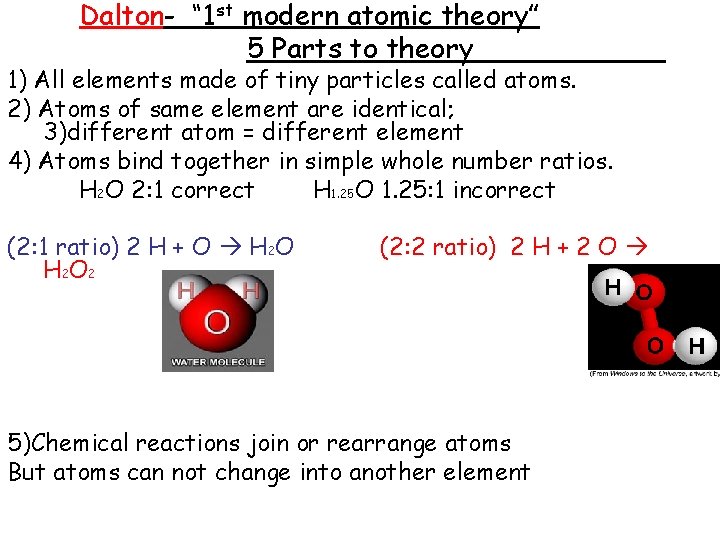
Dalton- “ 1 st modern atomic theory” 5 Parts to theory 1) All elements made of tiny particles called atoms. 2) Atoms of same element are identical; 3)different atom = different element 4) Atoms bind together in simple whole number ratios. H 2 O 2: 1 correct H 1. 25 O 1. 25: 1 incorrect (2: 1 ratio) 2 H + O H 2 O H 2 O 2 (2: 2 ratio) 2 H + 2 O 5)Chemical reactions join or rearrange atoms But atoms can not change into another element

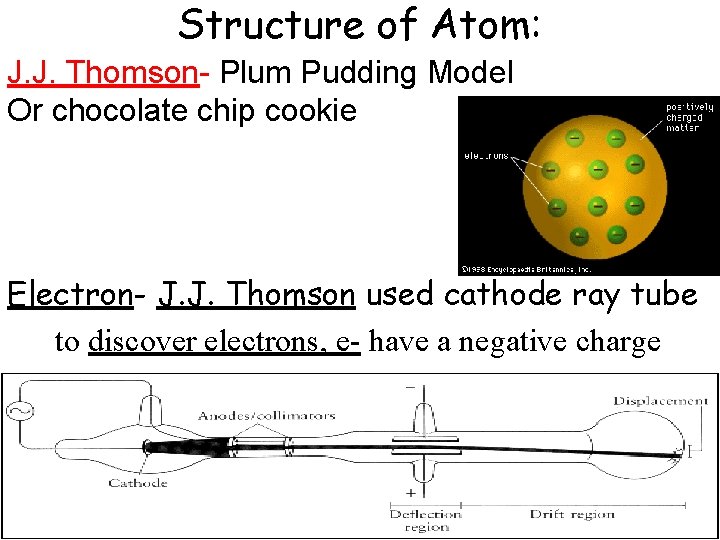
Structure of Atom: J. J. Thomson- Plum Pudding Model Or chocolate chip cookie Electron- J. J. Thomson used cathode ray tube to discover electrons, e- have a negative charge

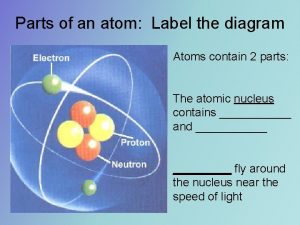
Structure of Atom: Proton- positively charged subatomic particle Goldstein- P+ mass 1840 times larger than e- mass Neutron- neutral charged subatomic particle - Discovered by Chadwick - Mass of proton = mass of neutron

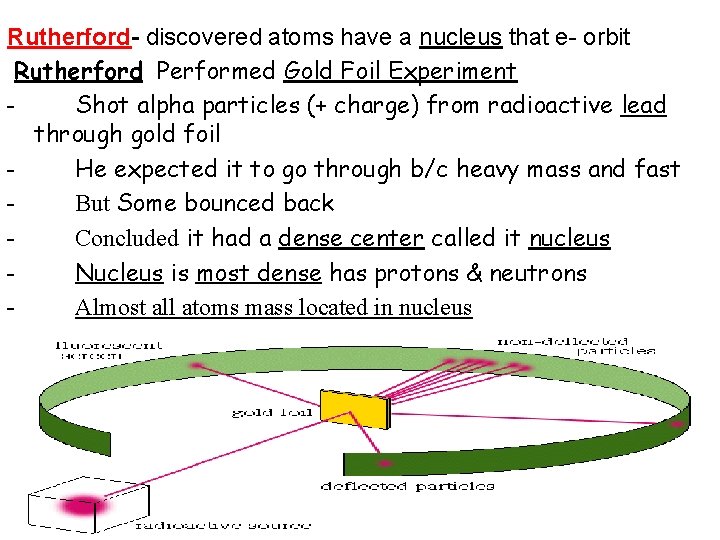
Rutherford- discovered atoms have a nucleus that e- orbit Rutherford Performed Gold Foil Experiment - Shot alpha particles (+ charge) from radioactive lead through gold foil - He expected it to go through b/c heavy mass and fast - But Some bounced back - Concluded it had a dense center called it nucleus - Nucleus is most dense has protons & neutrons - Almost all atoms mass located in nucleus

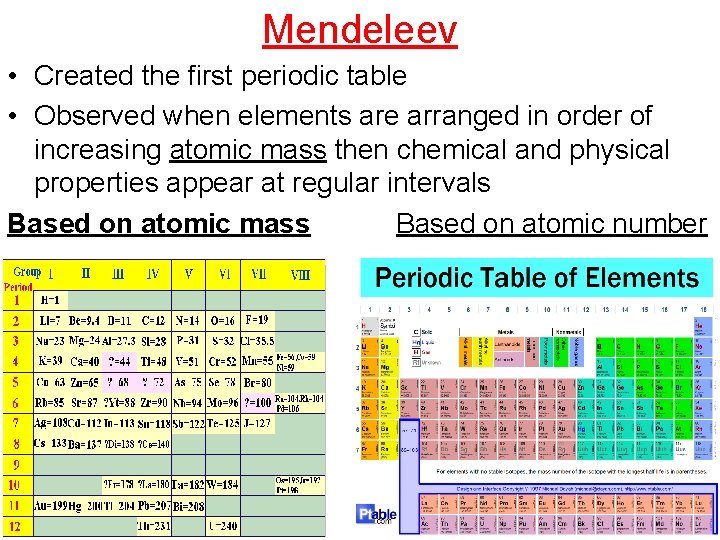
Mendeleev • Created the first periodic table • Observed when elements are arranged in order of increasing atomic mass then chemical and physical properties appear at regular intervals Based on atomic mass Based on atomic number

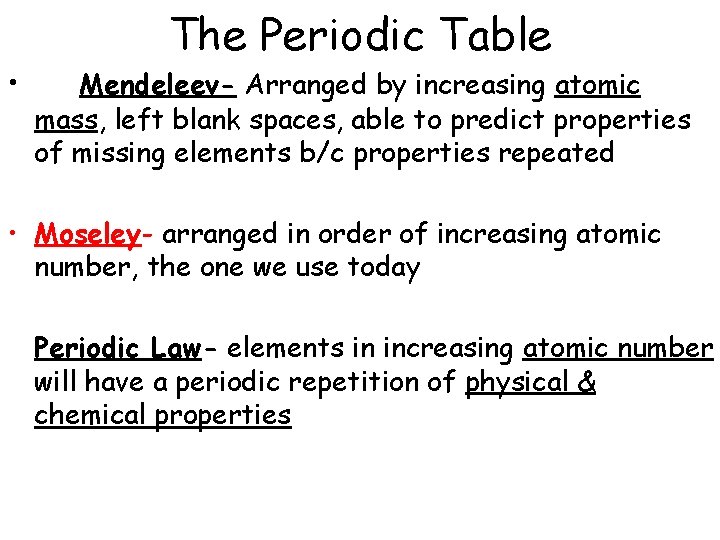
• The Periodic Table Mendeleev- Arranged by increasing atomic mass, left blank spaces, able to predict properties of missing elements b/c properties repeated • Moseley- arranged in order of increasing atomic number, the one we use today Periodic Law- elements in increasing atomic number will have a periodic repetition of physical & chemical properties

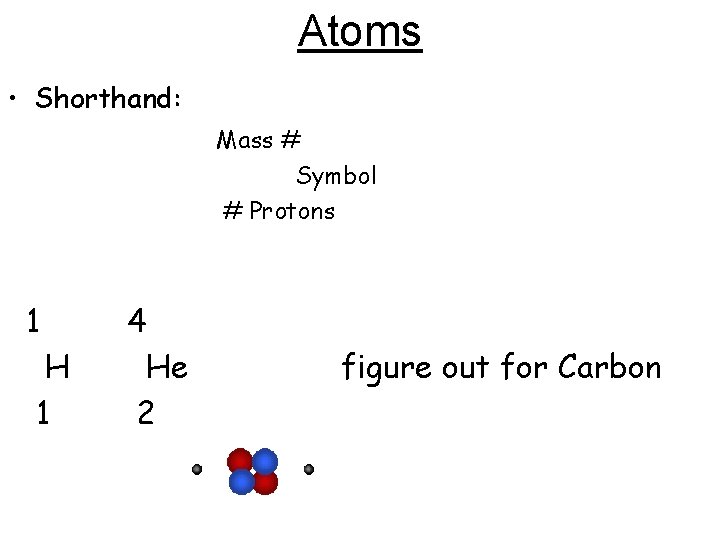
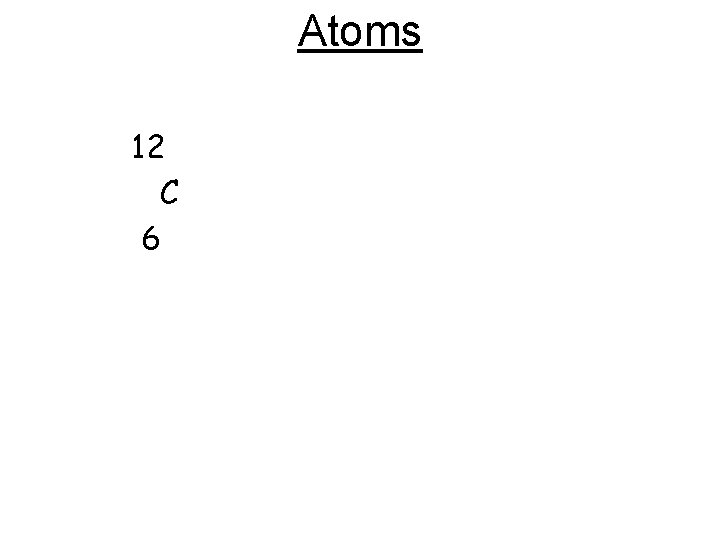
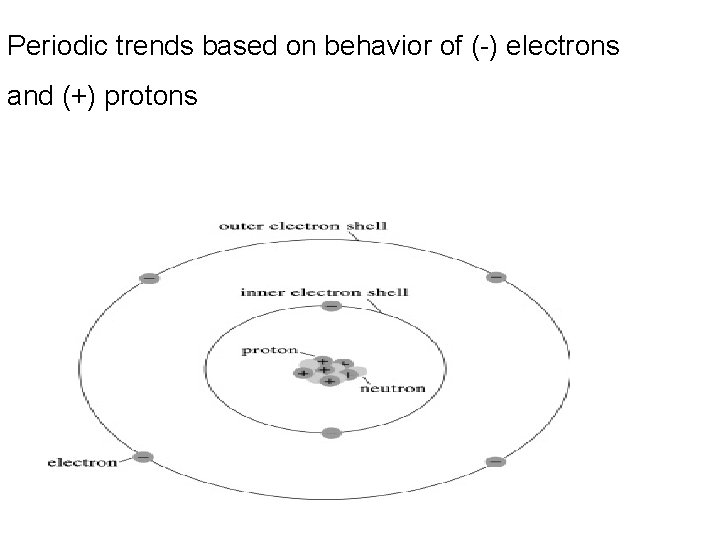
Atoms Atomic Number- # protons in nucleus Atoms are electrically neutral so #p = #e Protons = electrons

Atoms Mass Number = #p + #n = mass # number of protons + number of neutrons = mass number If given Mass # and proton # you can figure out #of neutrons Mass # - #p = #n

Atoms • Shorthand: Mass # Symbol # Protons 1 4 H 1 He 2 figure out for Carbon

Atoms 12 C 6

Atoms • Isotope- same # p, different # n; so different mass 1 2 H 1 3 H 1

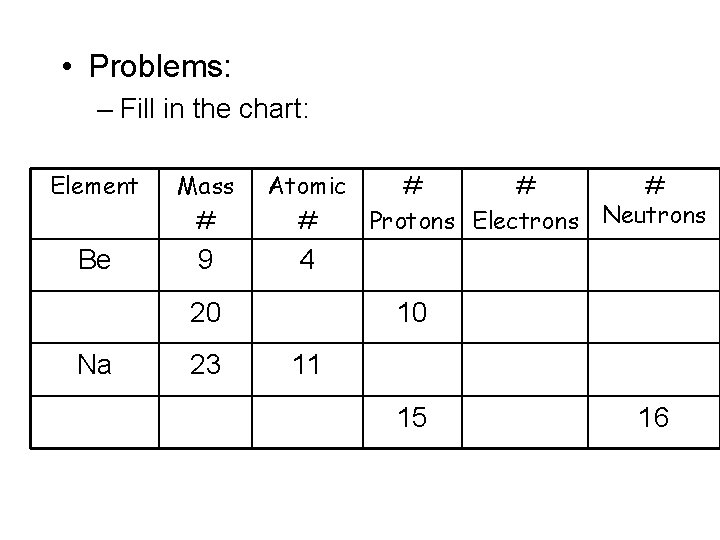
• Problems: – Fill in the chart: Element Mass # Be 9 Atomic # # Protons Electrons Neutrons 4 20 Na 23 10 11 15 16

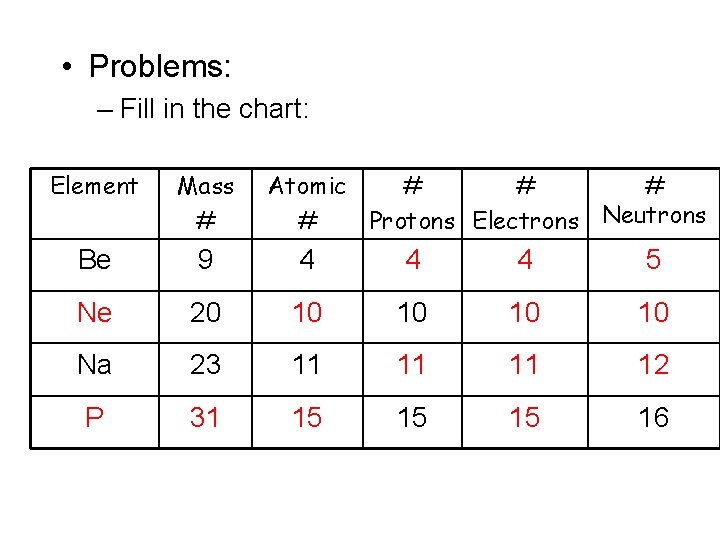
• Problems: – Fill in the chart: Element Mass # Atomic # # Protons Electrons Neutrons Be 9 4 4 4 5 Ne 20 10 10 Na 23 11 11 11 12 P 31 15 15 15 16

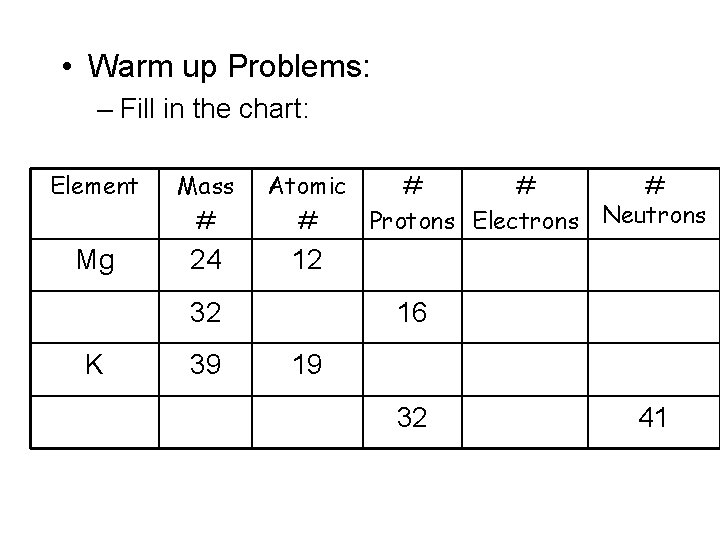
• Warm up Problems: – Fill in the chart: Element Mass # Mg 24 Atomic # # Protons Electrons Neutrons 12 32 K 39 16 19 32 41

• Warm up Answer: – Fill in the chart: Element Mass # Atomic # # Protons Electrons Neutrons Mg 24 12 12 S 32 16 16 K 39 19 19 19 20 Ge 73 32 32 32 41


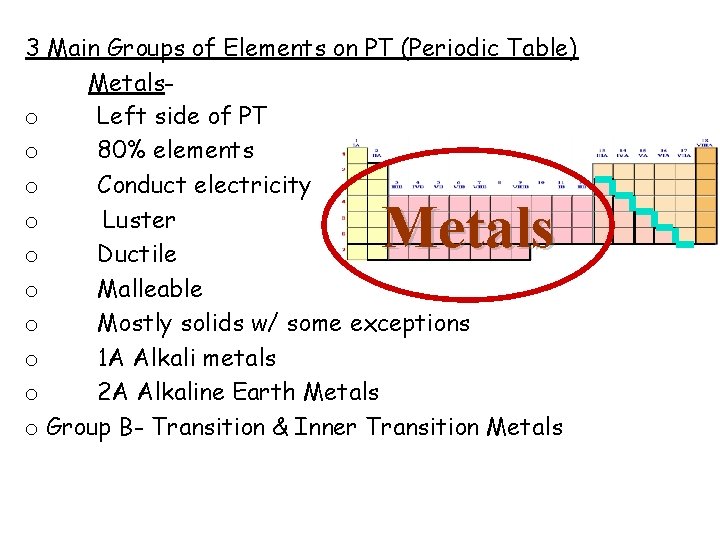
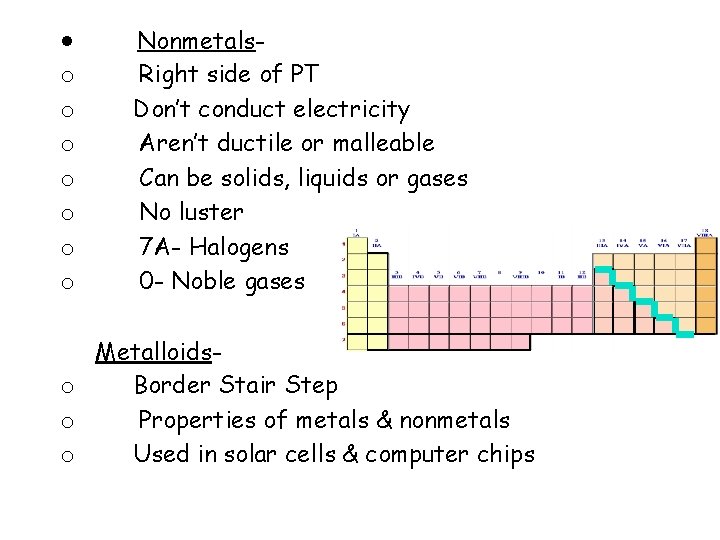
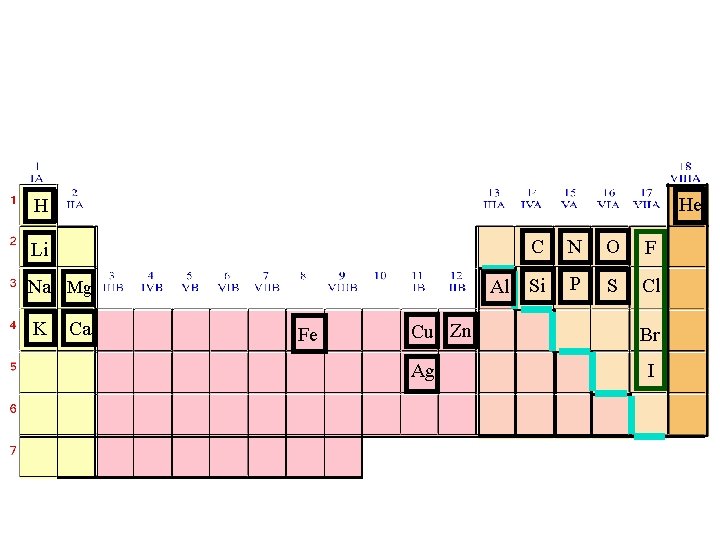
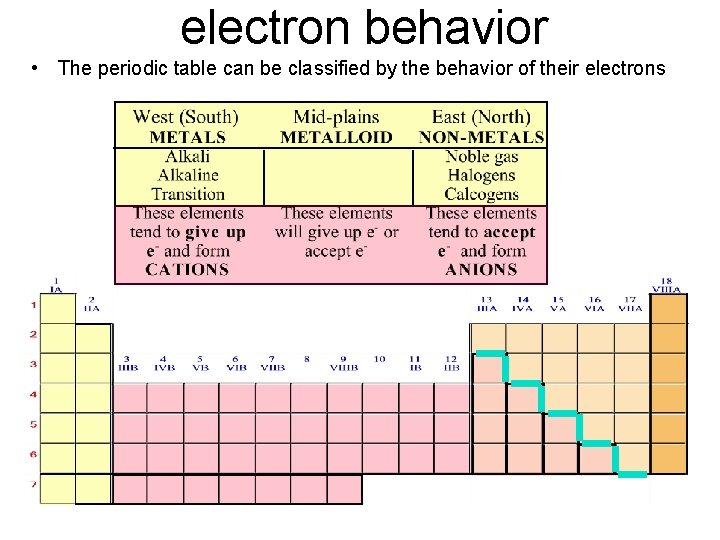
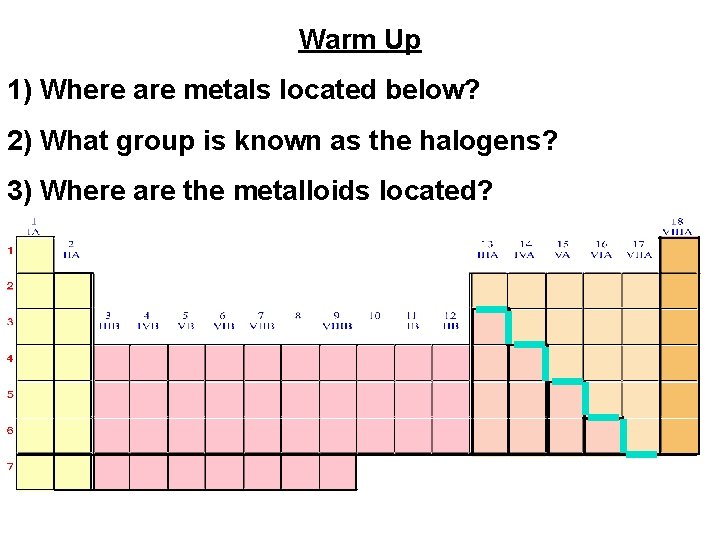
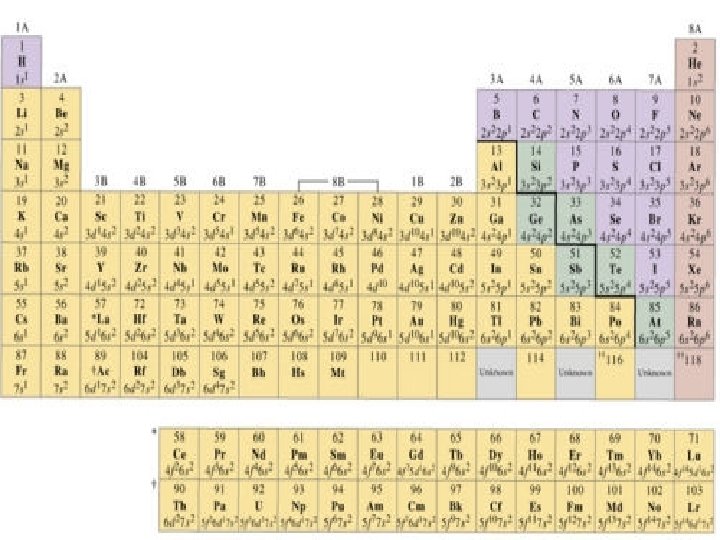
3 Main Groups of Elements on PT (Periodic Table) Metals Non-Metals Metalloids

3 Main Groups of Elements on PT (Periodic Table) Metalso Left side of PT o 80% elements o Conduct electricity o Luster o Ductile o Malleable o Mostly solids w/ some exceptions o 1 A Alkali metals o 2 A Alkaline Earth Metals o Group B- Transition & Inner Transition Metals

· Nonmetalso o Right side of PT Don’t conduct electricity Aren’t ductile or malleable Can be solids, liquids or gases No luster 7 A- Halogens 0 - Noble gases Metalloidso Border Stair Step o Properties of metals & nonmetals o Used in solar cells & computer chips

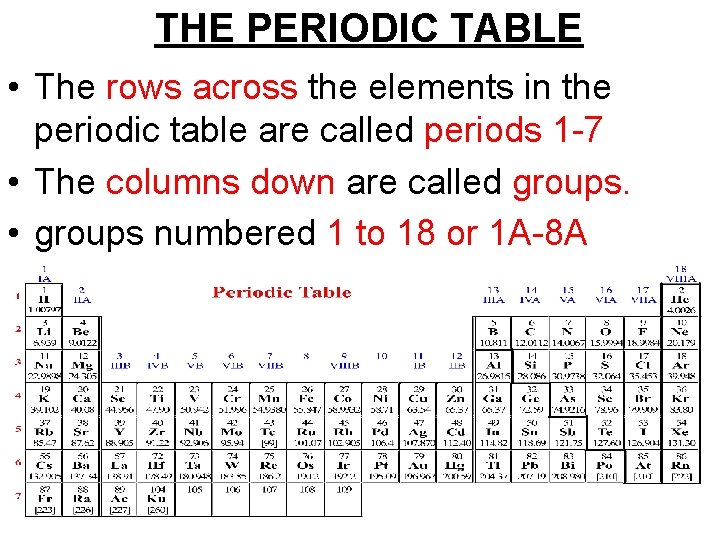
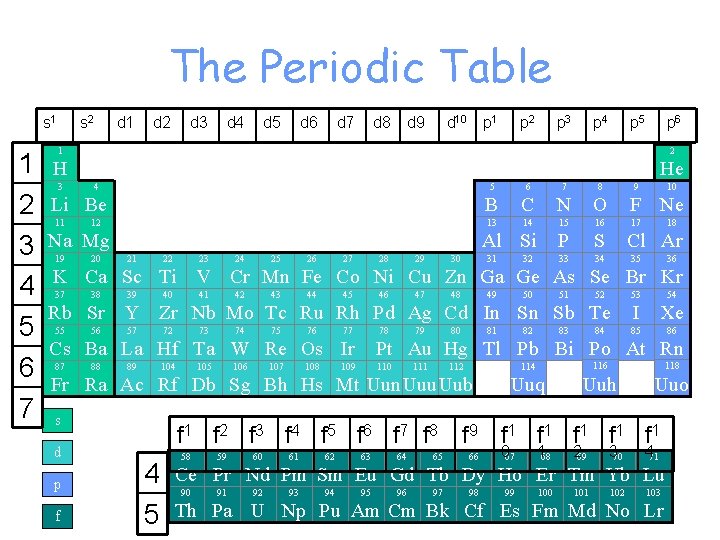
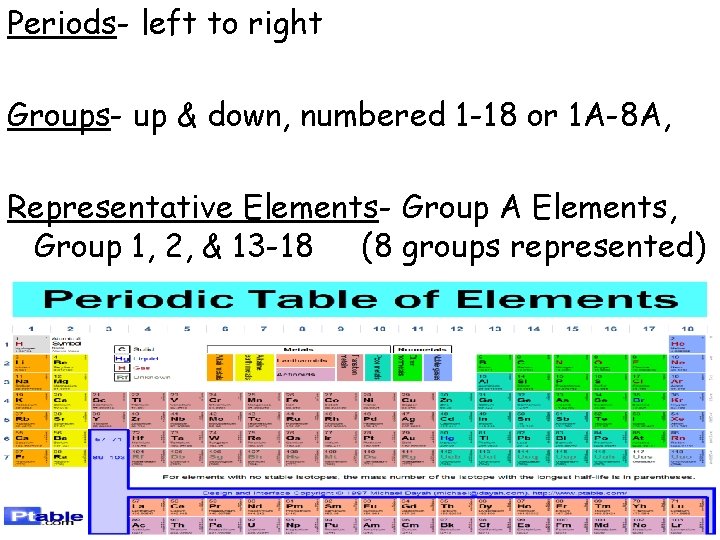
THE PERIODIC TABLE • The rows across the elements in the periodic table are called periods 1 -7 • The columns down are called groups. • groups numbered 1 to 18 or 1 A-8 A

GROUPS or Families • Group 1(1 A) are found on the far left side and are called the alkali metals • Group 2(2 A) are found on the far left side and are called the alkaline metals • Group 7 A(17) are found at the right hand side and are called the halogens. • Group 8 A(18) are called the noble gases

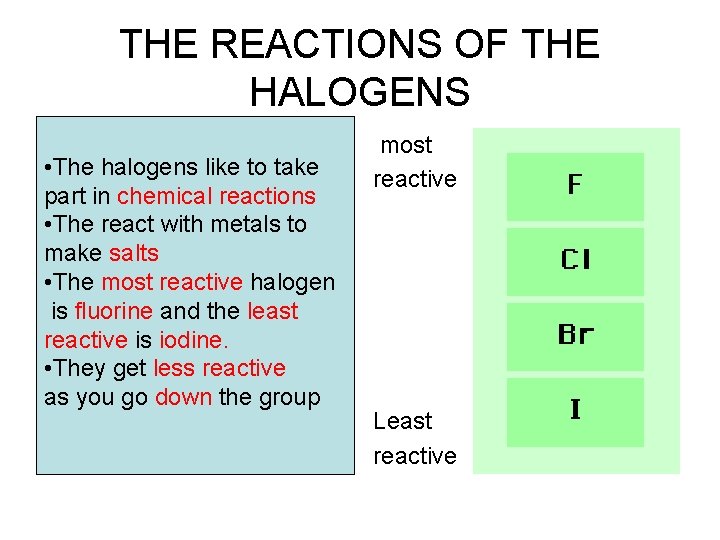
GROUP 7 - halogens are all non- metals they are: -Fluorine, Chlorine, Bromine, Iodine

• They have low melting points (this means that the change from a solid to a liquid at a low temperature) • They do not conduct electricity • They go around as pairs of atoms. • We call them diatomic molecules • We write them like this • Fluorine - F 2 • Chlorine - Cl 2 • Bromine - Br 2 • Iodine - I 2

What are they like? • • Fluorine is a very pale yellow gas Chlorine is a green gas Bromine is a dark red brown liquid Iodine is a black shiny solid

THE REACTIONS OF THE HALOGENS • The halogens like to take most • The halogens like to take part in chemical reactions reactive part in chemical reactions • The react with metals to make salts • • The most reactive halogen is fluorine and the least reactive is iodine. • They get less reactive as you go down the group Least reactive

Uses of the halogens • Iodine is used as an antiseptic • Chlorine is used in swimming pools to kill bacteria • Fluorine is used in tooth paste • Bromine is used photographic film

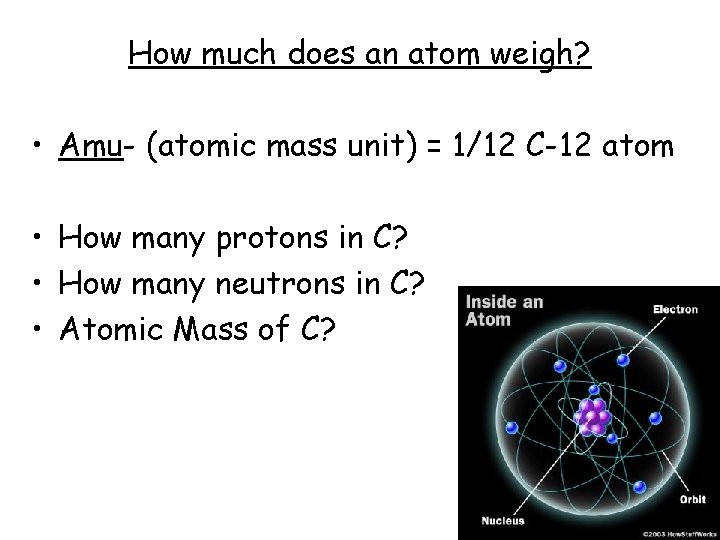
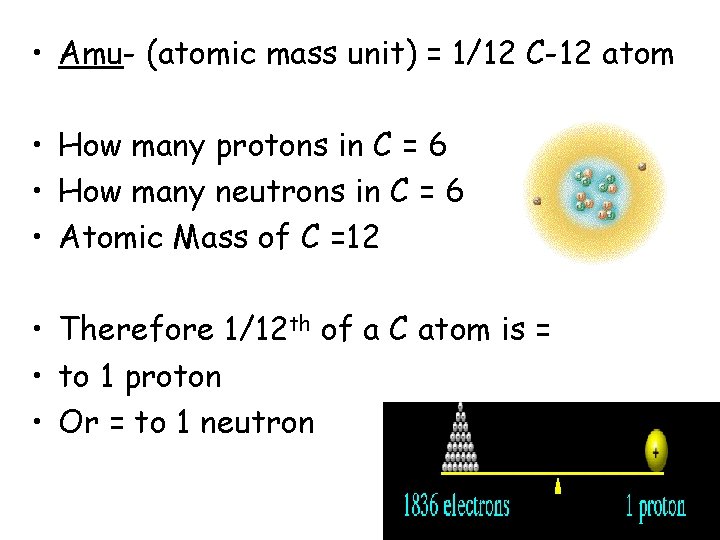
How much does an atom weigh? • Amu- (atomic mass unit) = 1/12 C-12 atom • How many protons in C? • How many neutrons in C? • Atomic Mass of C?

• Amu- (atomic mass unit) = 1/12 C-12 atom • How many protons in C = 6 • How many neutrons in C = 6 • Atomic Mass of C =12 • Therefore 1/12 th of a C atom is = • to 1 proton • Or = to 1 neutron

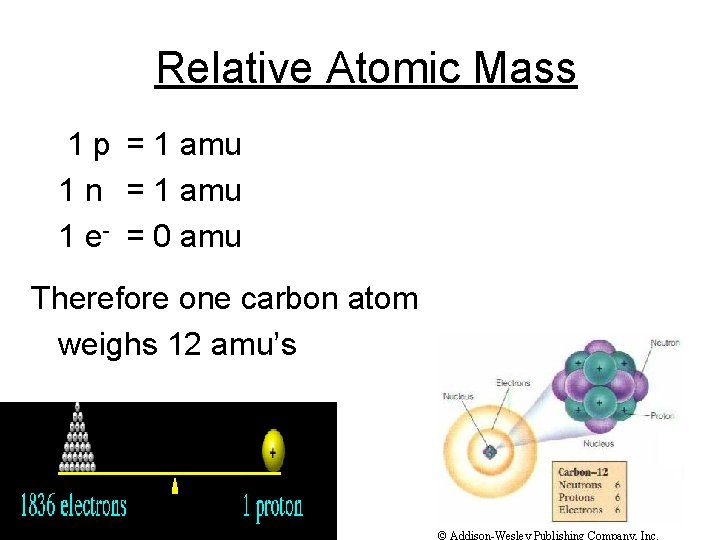
Relative Atomic Mass 1 p = 1 amu 1 n = 1 amu 1 e- = 0 amu Therefore one carbon atom weighs 12 amu’s © Addison-Wesley Publishing Company, Inc.

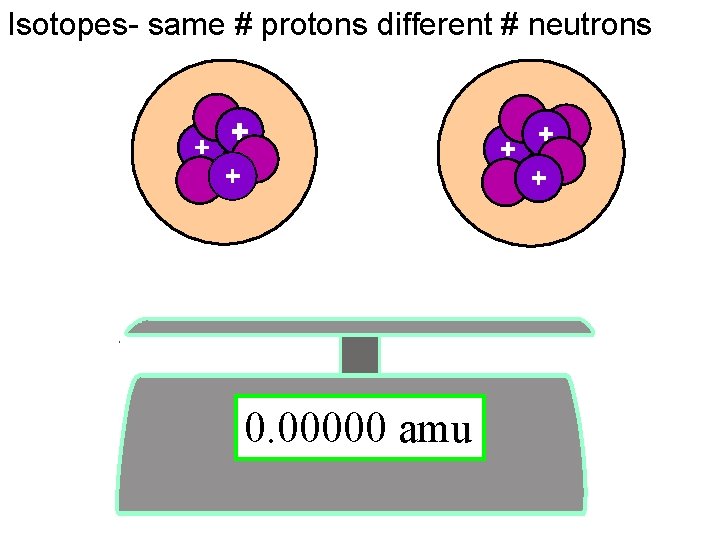
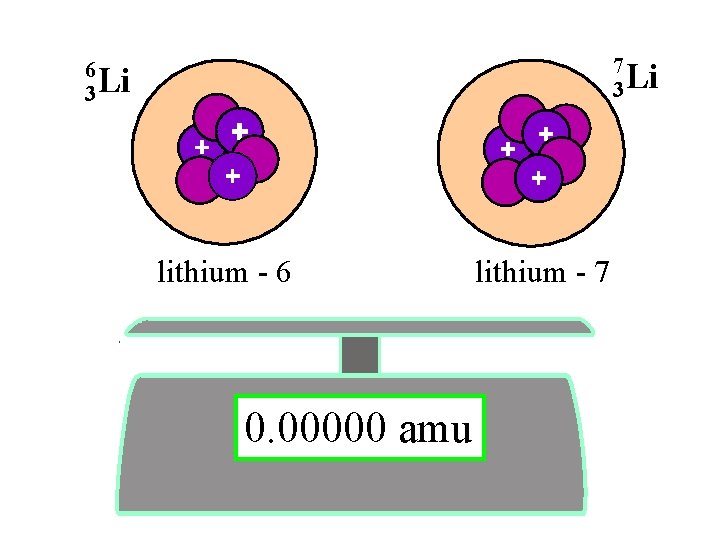
Isotopes- same # protons different # neutrons 6 3 7 3 Li 0. 00000 amu Li

6 3 7 3 Li lithium - 6 0. 00000 amu lithium - 7 Li

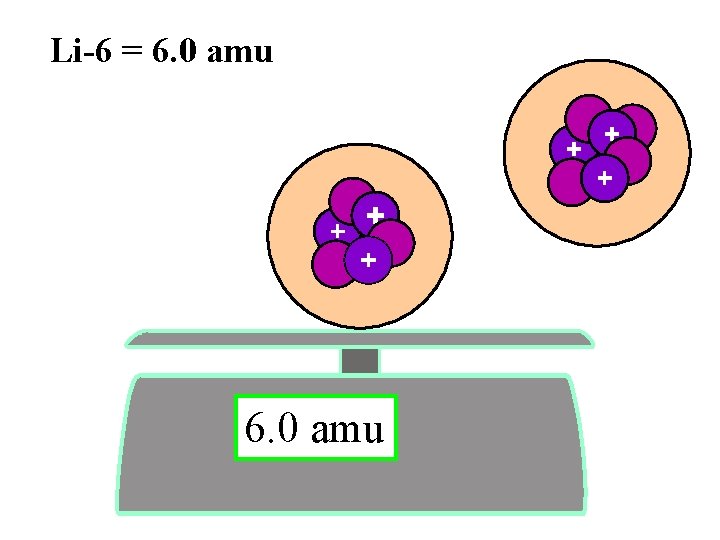
Li-6 = 6. 0 amu

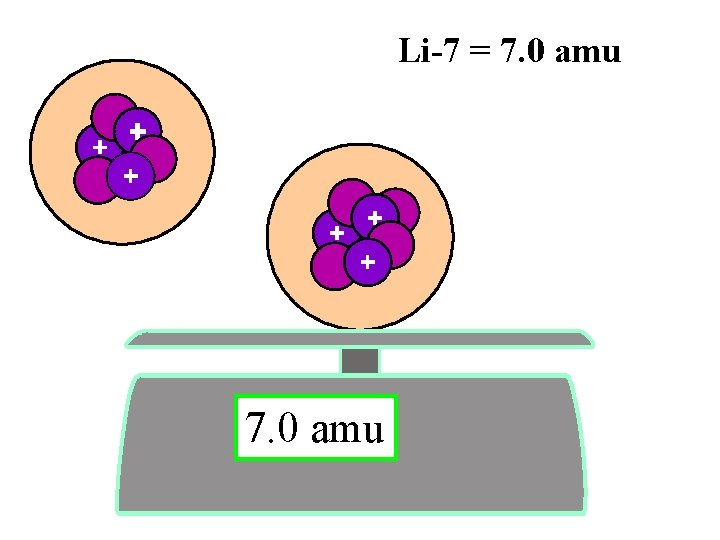
Li-7 = 7. 0 amu

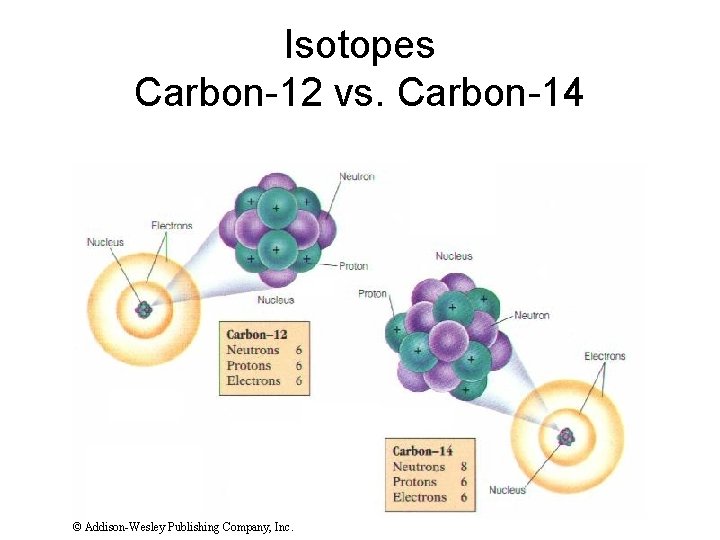
Isotopes Carbon-12 vs. Carbon-14 © Addison-Wesley Publishing Company, Inc.

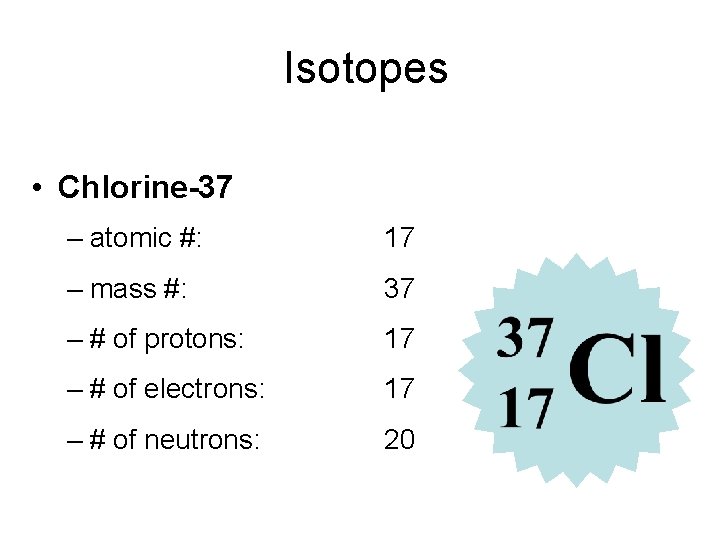
Isotopes • Chlorine-37 – atomic #: 17 – mass #: 37 – # of protons: 17 – # of electrons: 17 – # of neutrons: 20

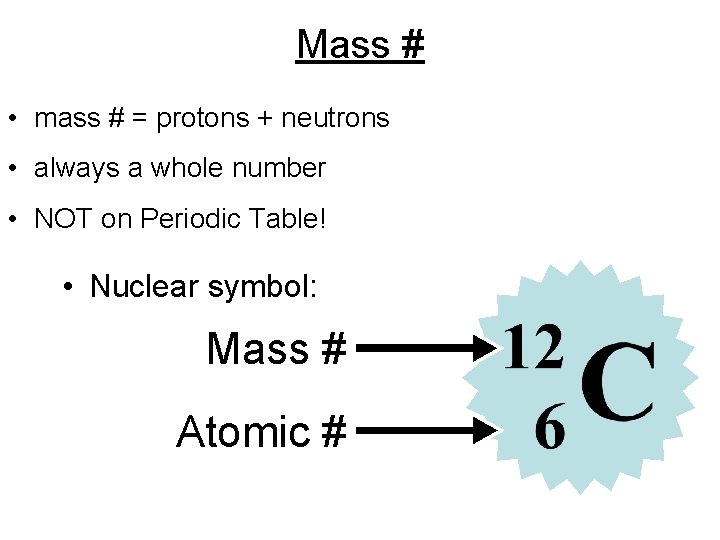
Mass # • mass # = protons + neutrons • always a whole number • NOT on Periodic Table! • Nuclear symbol: Mass # Atomic #

Average Atomic Mass • weighted average of all isotopes • on the Periodic Table • round to 2 decimal places

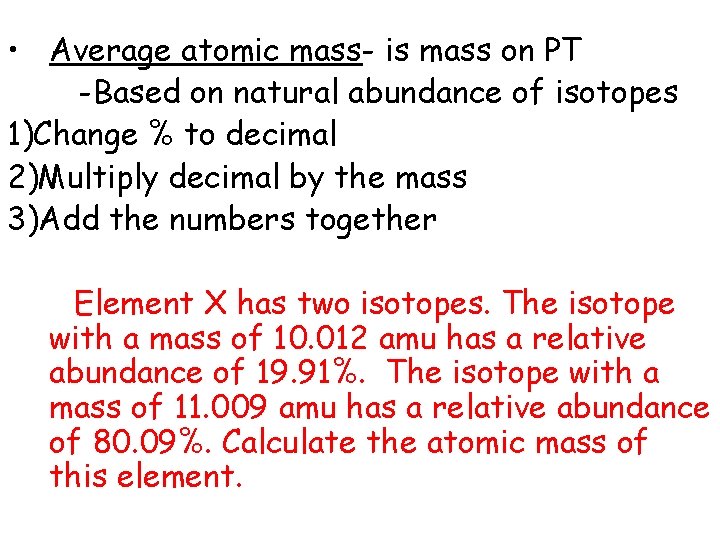
• Average atomic mass- is mass on PT -Based on natural abundance of isotopes 1. Change % to decimal 2. Multiply decimal by the mass 3. Add the numbers together

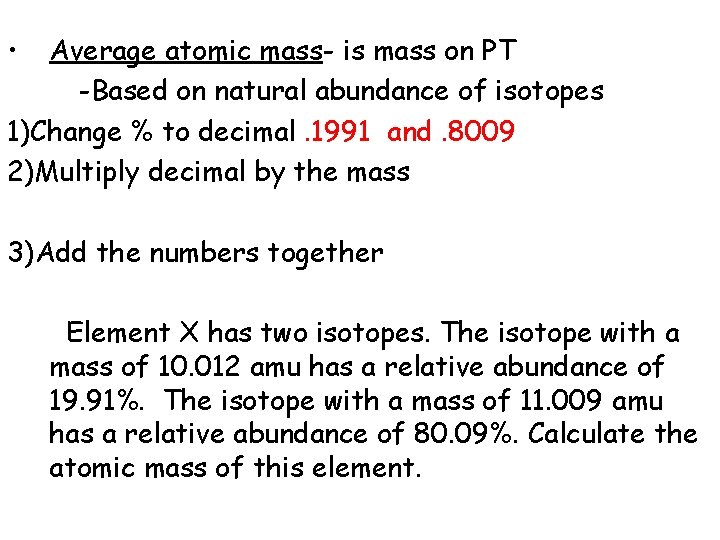
• Average atomic mass- is mass on PT -Based on natural abundance of isotopes 1)Change % to decimal 2)Multiply decimal by the mass 3)Add the numbers together Element X has two isotopes. The isotope with a mass of 10. 012 amu has a relative abundance of 19. 91%. The isotope with a mass of 11. 009 amu has a relative abundance of 80. 09%. Calculate the atomic mass of this element.

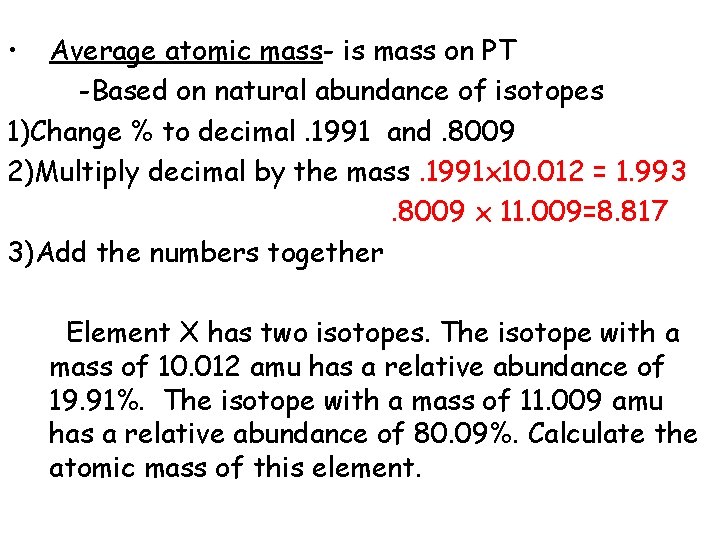
• Average atomic mass- is mass on PT -Based on natural abundance of isotopes 1)Change % to decimal. 1991 and. 8009 2)Multiply decimal by the mass 3)Add the numbers together Element X has two isotopes. The isotope with a mass of 10. 012 amu has a relative abundance of 19. 91%. The isotope with a mass of 11. 009 amu has a relative abundance of 80. 09%. Calculate the atomic mass of this element.

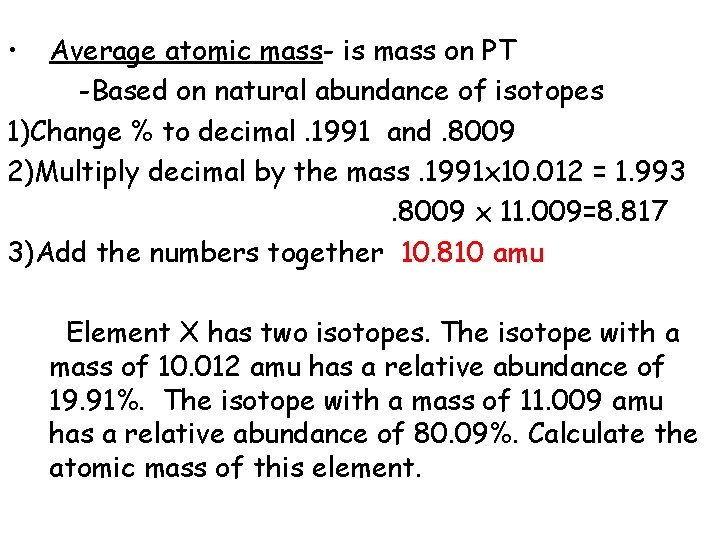
• Average atomic mass- is mass on PT -Based on natural abundance of isotopes 1)Change % to decimal. 1991 and. 8009 2)Multiply decimal by the mass. 1991 x 10. 012 = 1. 993. 8009 x 11. 009=8. 817 3)Add the numbers together Element X has two isotopes. The isotope with a mass of 10. 012 amu has a relative abundance of 19. 91%. The isotope with a mass of 11. 009 amu has a relative abundance of 80. 09%. Calculate the atomic mass of this element.

• Average atomic mass- is mass on PT -Based on natural abundance of isotopes 1)Change % to decimal. 1991 and. 8009 2)Multiply decimal by the mass. 1991 x 10. 012 = 1. 993. 8009 x 11. 009=8. 817 3)Add the numbers together 10. 810 amu Element X has two isotopes. The isotope with a mass of 10. 012 amu has a relative abundance of 19. 91%. The isotope with a mass of 11. 009 amu has a relative abundance of 80. 09%. Calculate the atomic mass of this element.
![Average atomic mass Worksheet Based on abundance of isotopes 1Change to decimal Average atomic mass [Worksheet] • Based on abundance of isotopes 1)Change % to decimal](https://slidetodoc.com/presentation_image_h/265ce1b76b2fa1010934e28daa8bdc65/image-44.jpg)
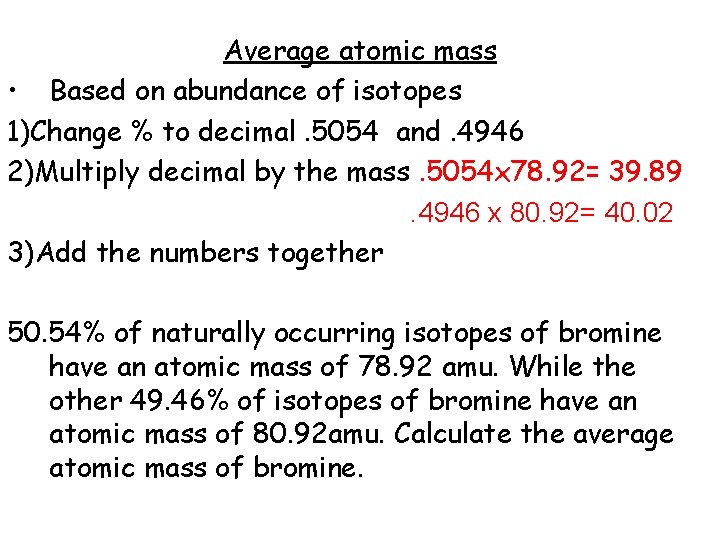
Average atomic mass [Worksheet] • Based on abundance of isotopes 1)Change % to decimal 2)Multiply decimal by the mass 3)Add the numbers together 50. 54% of naturally occurring isotopes of bromine have an atomic mass of 78. 92 amu. While the other 49. 46% of isotopes of bromine have an atomic mass of 80. 92 amu. Calculate the average atomic mass of bromine.

Average atomic mass • Based on abundance of isotopes 1)Change % to decimal. 5054 and. 4946 2)Multiply decimal by the mass 3)Add the numbers together 50. 54% of naturally occurring isotopes of bromine have an atomic mass of 78. 92 amu. While the other 49. 46% of isotopes of bromine have an atomic mass of 80. 92 amu. Calculate the average atomic mass of bromine.

Average atomic mass • Based on abundance of isotopes 1)Change % to decimal. 5054 and. 4946 2)Multiply decimal by the mass. 5054 x 78. 92= 39. 89 . 4946 x 80. 92= 40. 02 3)Add the numbers together 50. 54% of naturally occurring isotopes of bromine have an atomic mass of 78. 92 amu. While the other 49. 46% of isotopes of bromine have an atomic mass of 80. 92 amu. Calculate the average atomic mass of bromine.

Average atomic mass • Based on abundance of isotopes 1)Change % to decimal. 5054 and. 4946 2)Multiply decimal by the mass. 5054 x 78. 92= 39. 89 . 4946 x 80. 92= 40. 02 3)Add the numbers together 39. 89 + 40. 02 = 79. 91 50. 54% of naturally occurring isotopes of bromine have an atomic mass of 78. 92 amu. While the other 49. 46% of isotopes of bromine have an atomic mass of 80. 92 amu. Calculate the average atomic mass of bromine.

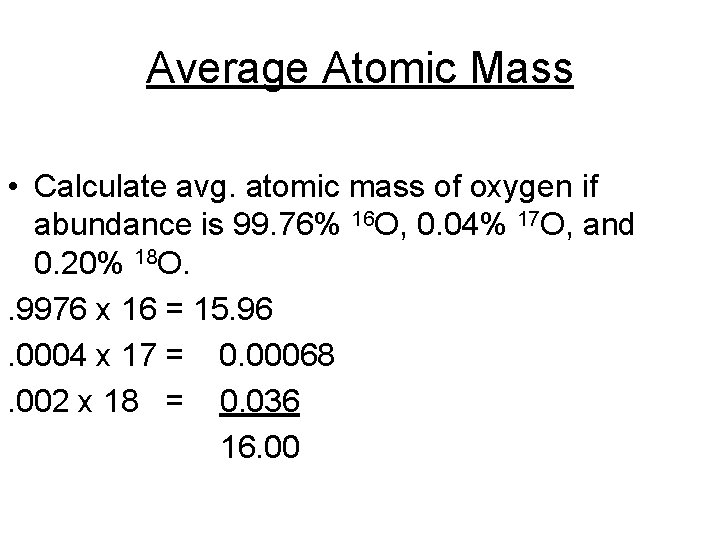
Average Atomic Mass • Calculate avg. atomic mass of oxygen if abundance is 99. 76% 16 O, 0. 04% 17 O, and 0. 20% 18 O. . 9976 x 16 = 15. 96. 0004 x 17 = 0. 00068 16. 002 x 18 = 0. 036 amu 16. 00

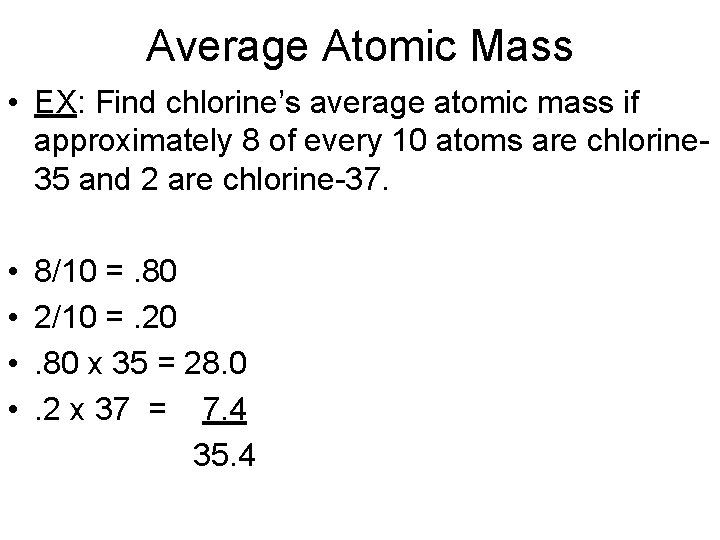
Average Atomic Mass • EX: Find chlorine’s average atomic mass if approximately 8 of every 10 atoms are chlorine 35 and 2 are chlorine-37. • 8/10 =. 80 • 2/10 =. 20 • . 80 x 35 = 28. 0 • . 2 x 37 = 7. 4 35. 40 amu

• Warm up Answer: – Fill in the chart: Element Mass # Atomic # # Protons Electrons Neutrons Mg 24 12 12 S 32 16 16 K 39 19 19 19 20 Ge 73 32 32 32 41

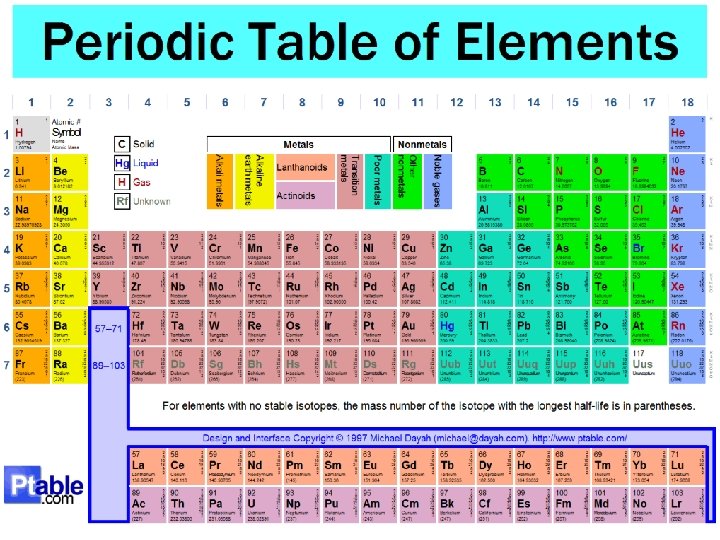
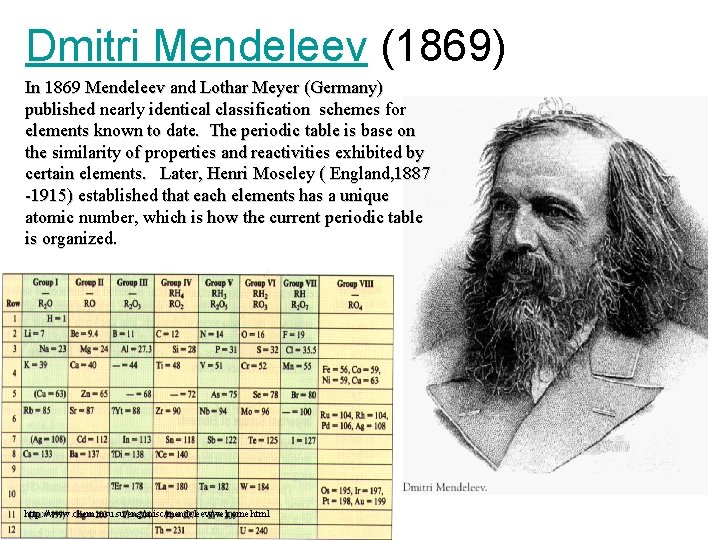
Dmitri Mendeleev (1869) In 1869 Mendeleev and Lothar Meyer (Germany) published nearly identical classification schemes for elements known to date. The periodic table is base on the similarity of properties and reactivities exhibited by certain elements. Later, Henri Moseley ( England, 1887 -1915) established that each elements has a unique atomic number, which is how the current periodic table is organized. http: //www. chem. msu. su/eng/misc/mendeleev/welcome. html

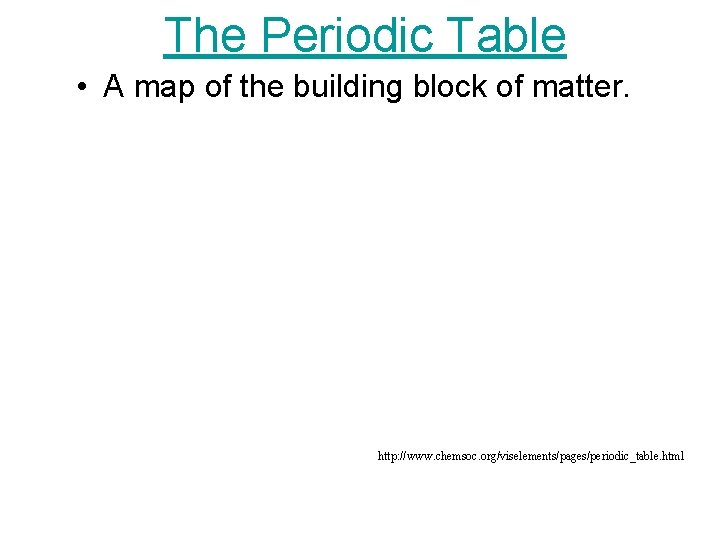
The Periodic Table • A map of the building block of matter. http: //www. chemsoc. org/viselements/pages/periodic_table. html

Periodic Table: Metallic arrangement • Layout of the Periodic Table: Metals vs. nonmetals Metals Nonmetals

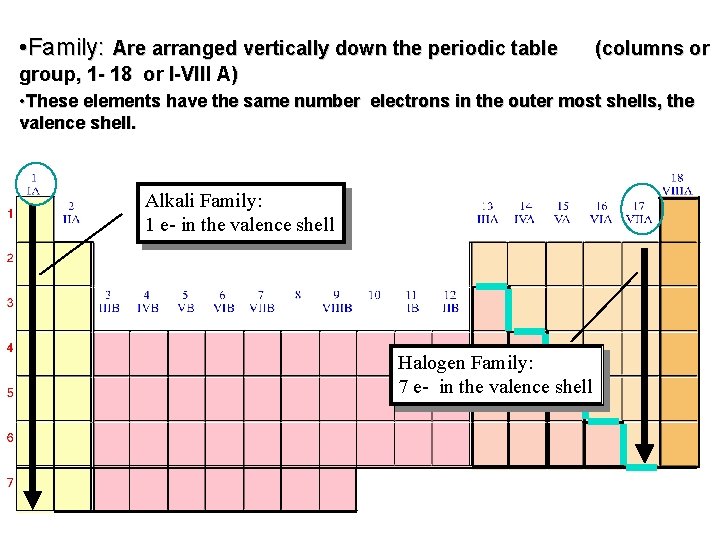
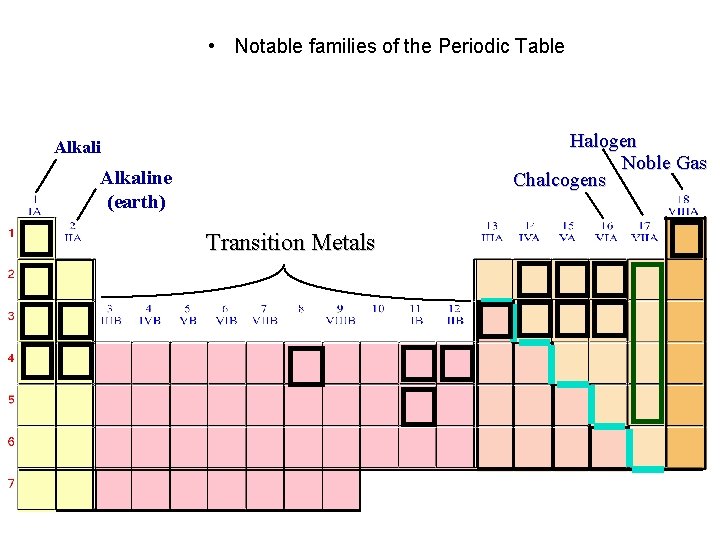
• Family: Are arranged vertically down the periodic table (columns or group, 1 - 18 or I-VIII A) • These elements have the same number electrons in the outer most shells, the valence shell. Alkali Family: 1 e- in the valence shell Halogen Family: 7 e- in the valence shell

• Notable families of the Periodic Table Halogen Noble Gas Chalcogens Alkaline (earth) Transition Metals

He H Li Na Mg K Ca Fe Cu Zn Ag C N O F Al Si P S Cl Br I

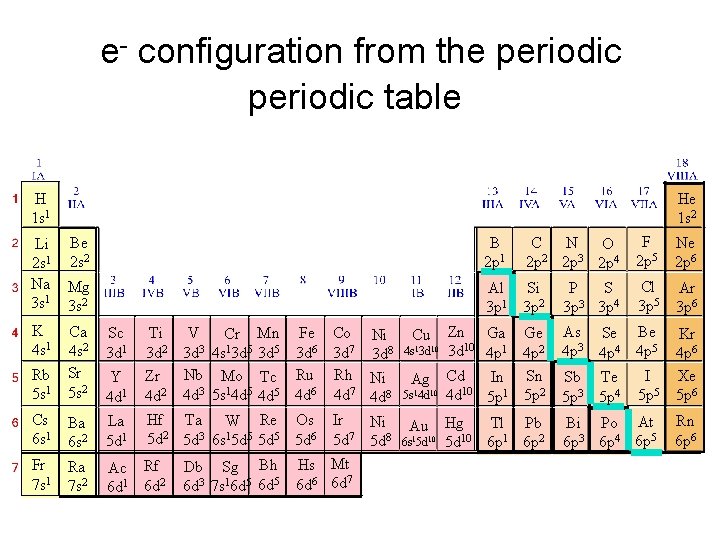
e- configuration from the periodic table H 1 s 1 He 1 s 2 Li Be 2 s 1 2 s 2 Na Mg 3 s 1 3 s 2 K 4 s 1 Sc 3 d 1 Rb 5 s 1 Ca 4 s 2 Sr 5 s 2 Y 4 d 1 V Ti Cr Mn Fe Co 3 d 2 3 d 3 4 s 13 d 5 3 d 6 3 d 7 Zr Nb Mo Tc Ru Rh 4 d 2 4 d 3 5 s 14 d 5 4 d 6 4 d 7 Cs 6 s 1 Ba 6 s 2 La 5 d 1 Hf Ta W Re Os 5 d 2 5 d 3 6 s 15 d 5 5 d 6 Fr 7 s 1 Ra 7 s 2 Ac Rf 6 d 1 6 d 2 Db Sg Bh 6 d 3 7 s 16 d 5 Ni 3 d 8 Ni 4 d 8 Ir Ni 7 5 d 5 d 8 Hs Mt 6 d 6 6 d 7 Cu 4 s 13 d 10 Ag 5 s 14 d 10 Au 6 s 15 d 10 B 2 p 1 • C B N O 1 2 3 • 2 p 2 p 4 F 2 p 5 Ne 2 p 6 Al 3 p 1 Si 3 p 2 S P 3 3 p 3 p 4 Cl 3 p 5 Ar 3 p 6 Zn Ga Ge 3 d 10 4 p 1 4 p 2 Cd In Sn 10 4 d 5 p 1 5 p 2 As Se 4 p 3 4 p 4 Be 4 p 5 Sb Te 5 p 3 5 p 4 I 5 p 5 Kr 4 p 6 Xe 5 p 6 Hg Tl Pb 5 d 10 6 p 1 6 p 2 Bi Po At 6 p 3 6 p 4 6 p 5 Rn 6 p 6

electron behavior • The periodic table can be classified by the behavior of their electrons

Warm Up 1) Where are metals located below? 2) What group is known as the halogens? 3) Where are the metalloids located?

Dmitri Mendeleev (1869) Based on atomic mass Today's periodic table organized by? http: //www. chem. msu. su/eng/misc/mendeleev/welcome. html

Periodic Trends • Today we will rationalize observed trends in – Atomic radius – Ionization energy. – Electronegativity

Periodic trends based on behavior of (-) electrons and (+) protons

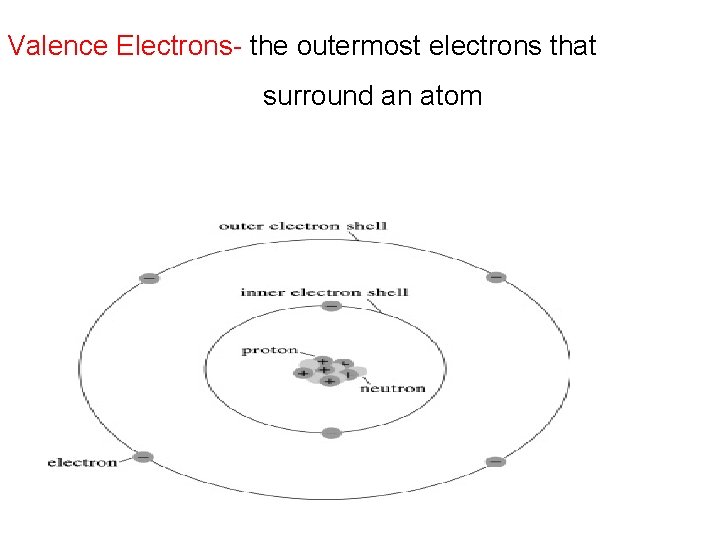
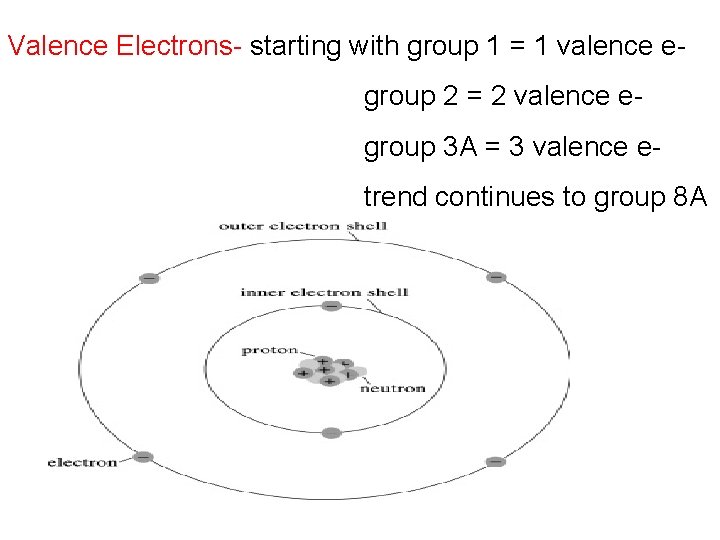
Valence Electrons- the outermost electrons that surround an atom

Valence Electrons- starting with group 1 = 1 valence e group 2 = 2 valence e group 3 A = 3 valence e- trend continues to group 8 A

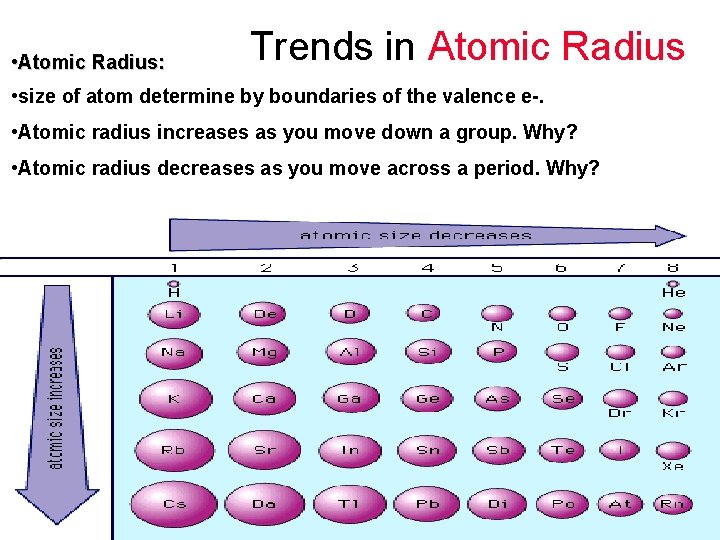
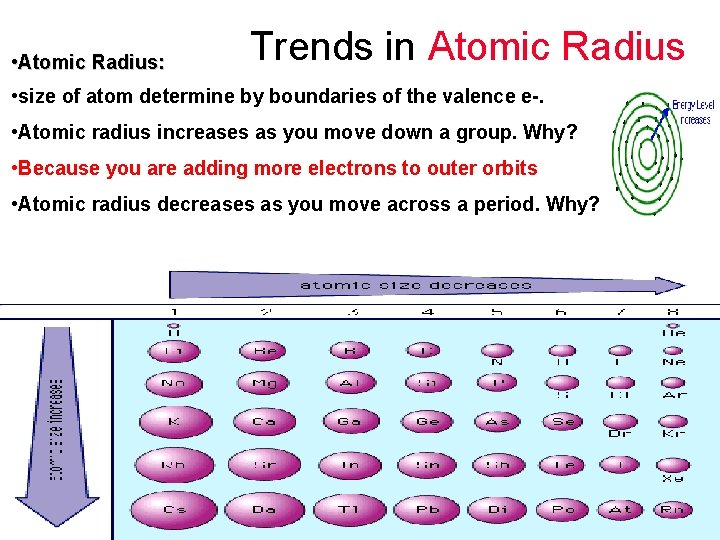
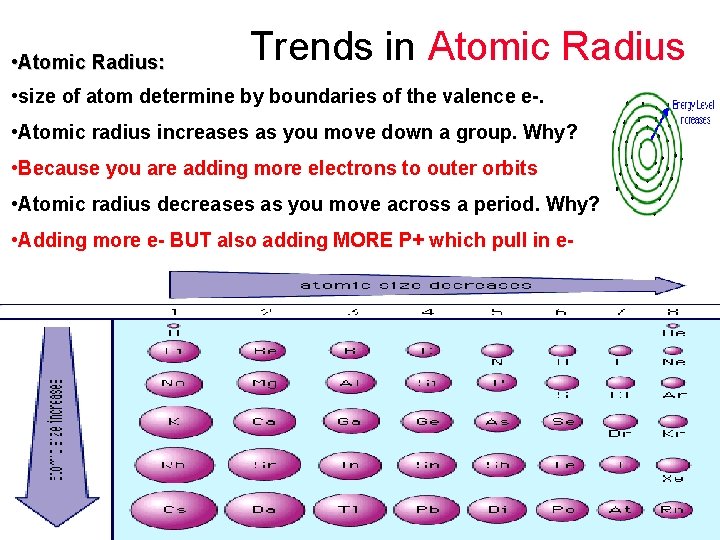
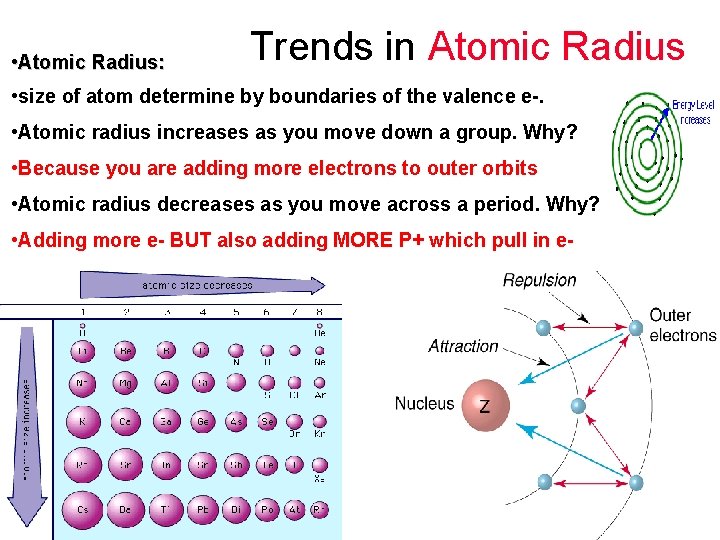
Trends in Atomic Radius • Atomic Radius: • size of atom determine by boundaries of the valence e-. • Atomic radius increases as you move down a group. Why? • Atomic radius decreases as you move across a period. Why?

Trends in Atomic Radius • Atomic Radius: • size of atom determine by boundaries of the valence e-. • Atomic radius increases as you move down a group. Why? • Because you are adding more electrons to outer orbits • Atomic radius decreases as you move across a period. Why?

Trends in Atomic Radius • Atomic Radius: • size of atom determine by boundaries of the valence e-. • Atomic radius increases as you move down a group. Why? • Because you are adding more electrons to outer orbits • Atomic radius decreases as you move across a period. Why? • Adding more e- BUT also adding MORE P+ which pull in e-

Trends in Atomic Radius • Atomic Radius: • size of atom determine by boundaries of the valence e-. • Atomic radius increases as you move down a group. Why? • Because you are adding more electrons to outer orbits • Atomic radius decreases as you move across a period. Why? • Adding more e- BUT also adding MORE P+ which pull in e-

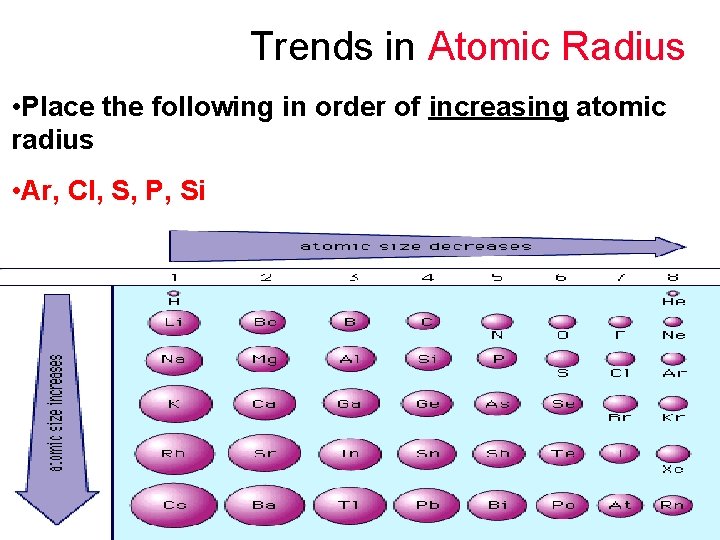
Trends in Atomic Radius • Place the following in order of increasing atomic radius • S, P, Ar, Cl, Si

Trends in Atomic Radius • Place the following in order of increasing atomic radius • Ar, Cl, S, P, Si

Trends in Atomic Radius • Atomic Radius:

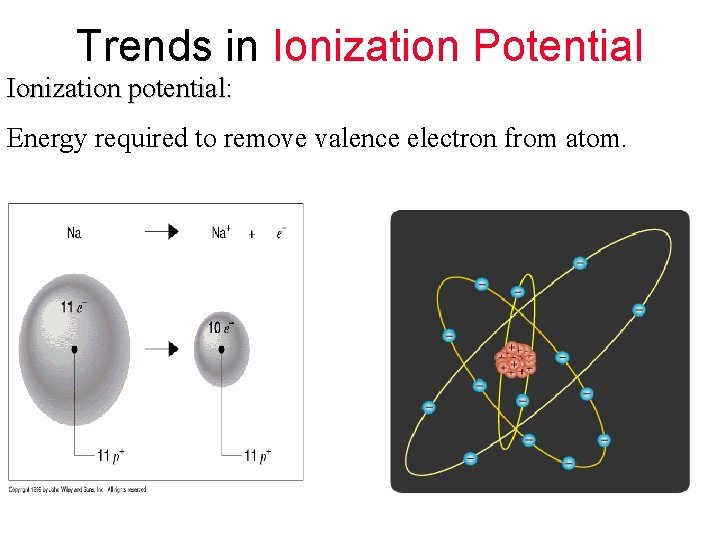
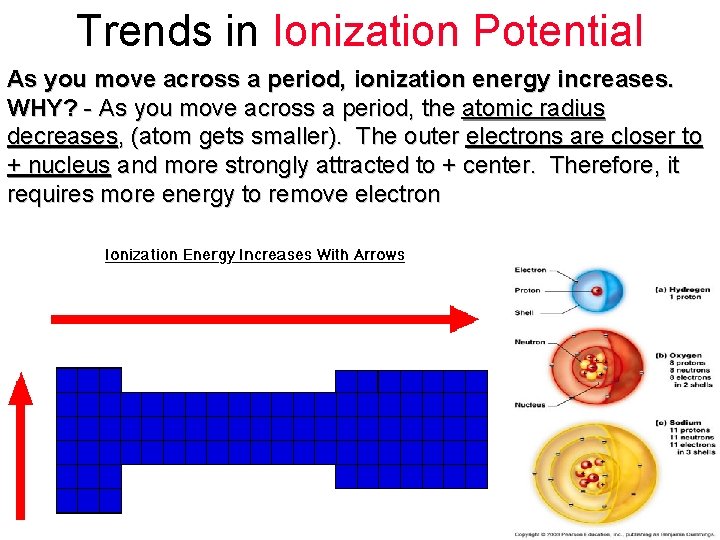
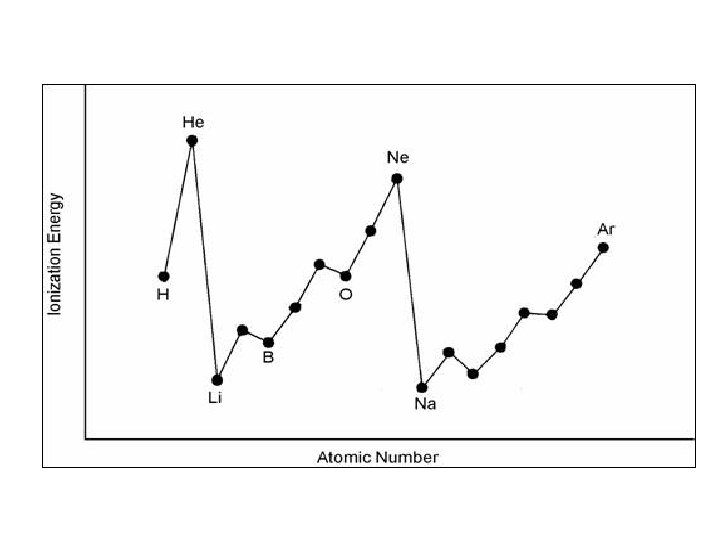
Trends in Ionization Potential Ionization potential: Energy required to remove valence electron from atom.

Trends in Ionization Potential As you move across a period, ionization energy increases. WHY? - As you move across a period, the atomic radius decreases, (atom gets smaller). The outer electrons are closer to + nucleus and more strongly attracted to + center. Therefore, it requires more energy to remove electron

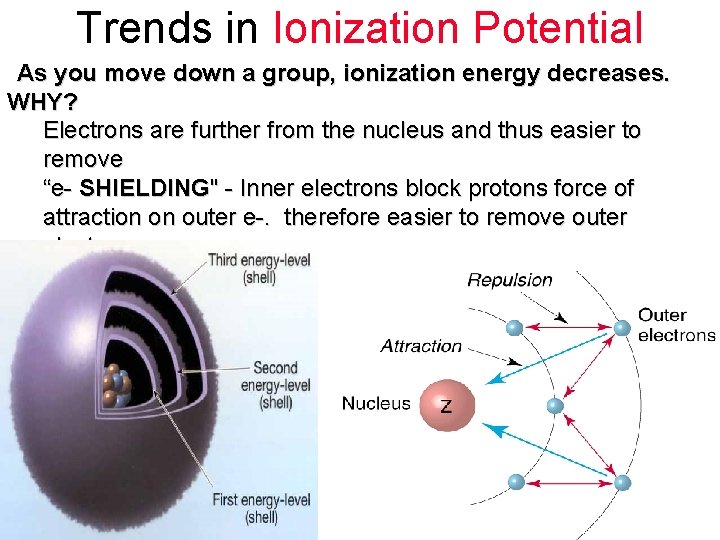
Trends in Ionization Potential As you move down a group, ionization energy decreases. WHY? Electrons are further from the nucleus and thus easier to remove “e- SHIELDING" - Inner electrons block protons force of attraction on outer e-. therefore easier to remove outer electron

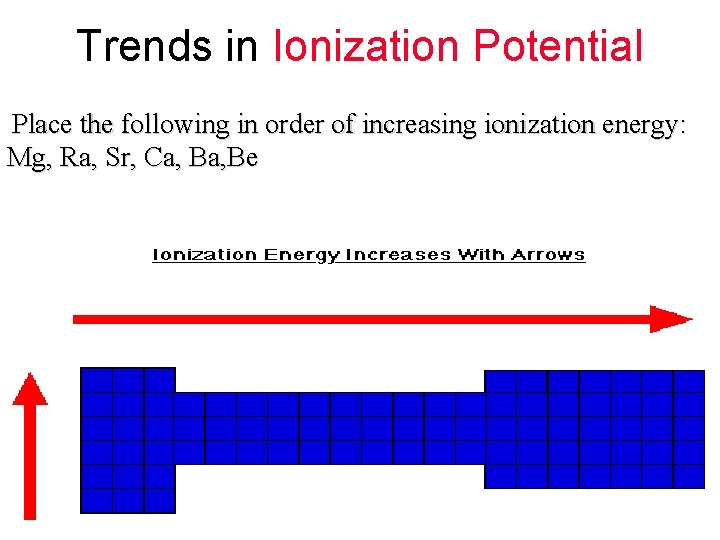
Trends in Ionization Potential Place the following in order of increasing ionization energy: Mg, Ra, Sr, Ca, Be

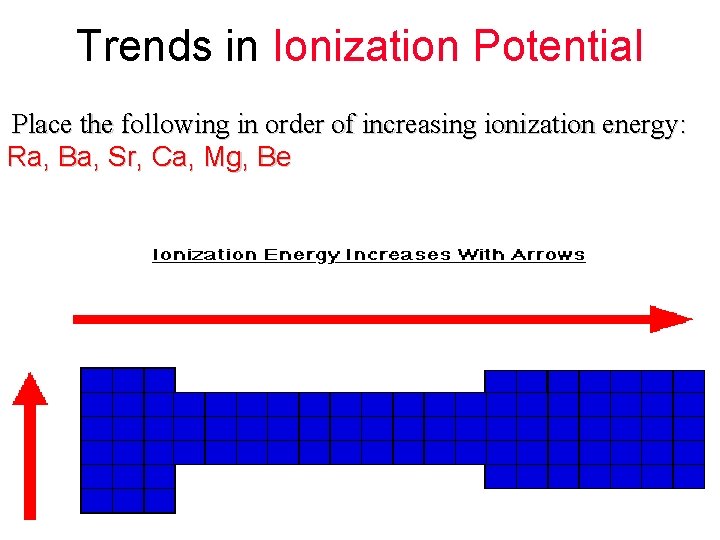
Trends in Ionization Potential Place the following in order of increasing ionization energy: Ra, Ba, Sr, Ca, Mg, Be

Trends in Electronegativity The ability of an atom to attract (steal) electrons to itself:

Trends in Electronegativity The ability of an atom to attract (steal) electrons to itself: Which is more electronegative? K or S and why?

Trends in Electronegativity The ability of an atom to attract (steal) electrons to itself: Which is more electronegative? S why? b/c small radius of atom means the + nucleus can attract the –e from other atoms

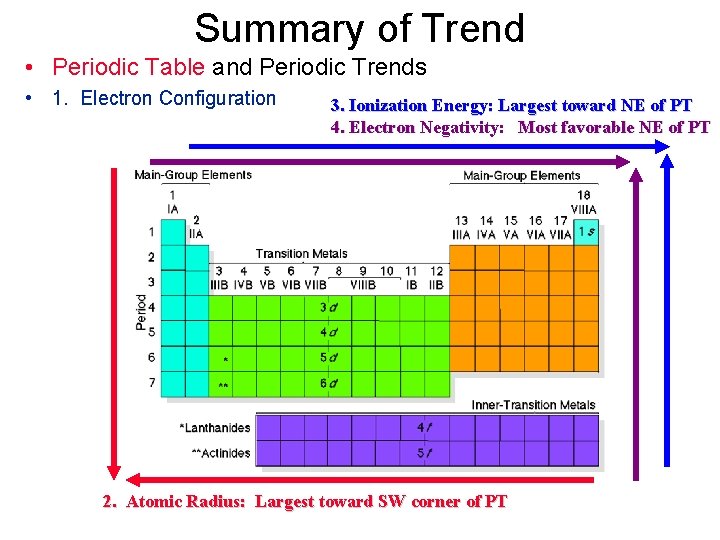
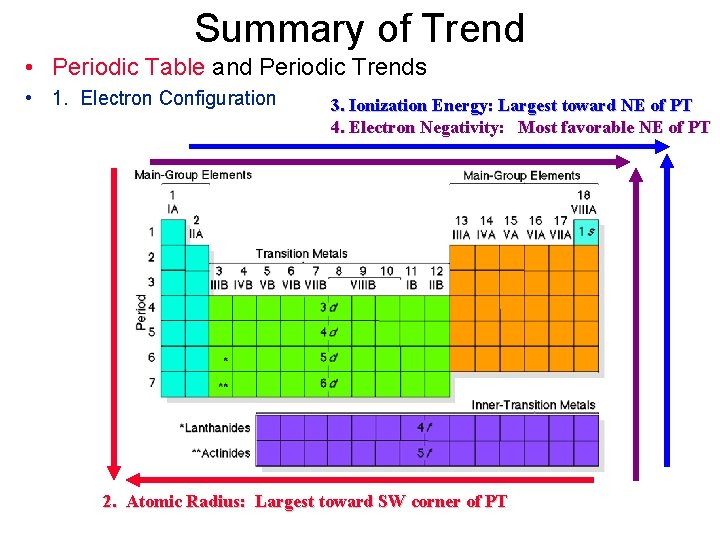
Summary of Trend • Periodic Table and Periodic Trends • 1. Electron Configuration 3. Ionization Energy: Largest toward NE of PT 4. Electron Negativity: Most favorable NE of PT 2. Atomic Radius: Largest toward SW corner of PT

Periodic Table Patterns • Elements in the same group generally have similar chemical properties. • Properties are not identical, however.

Periodic Table Patterns Dmitri Mendeleev came to the conclusion that Elements in the same group generally have similar chemical properties.

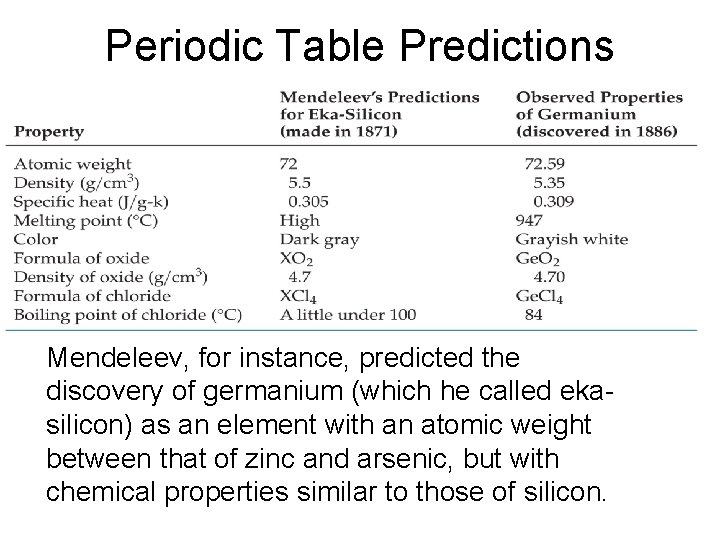
Periodic Table Predictions Mendeleev, for instance, predicted the discovery of germanium (which he called ekasilicon) as an element with an atomic weight between that of zinc and arsenic, but with chemical properties similar to those of silicon.


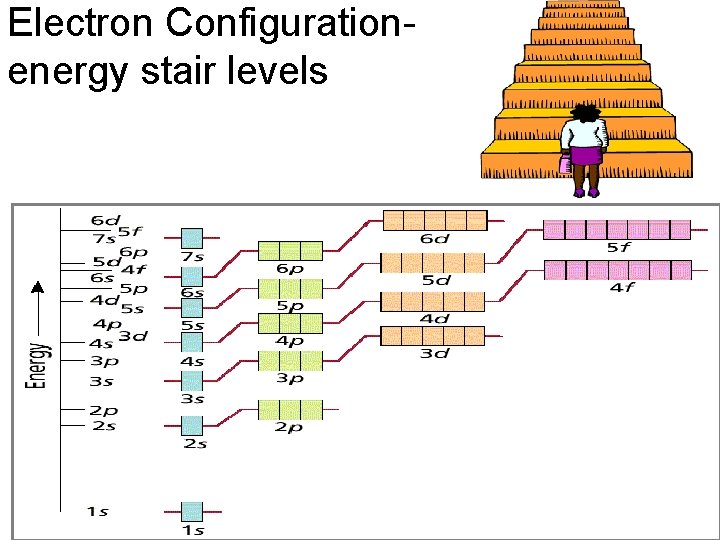
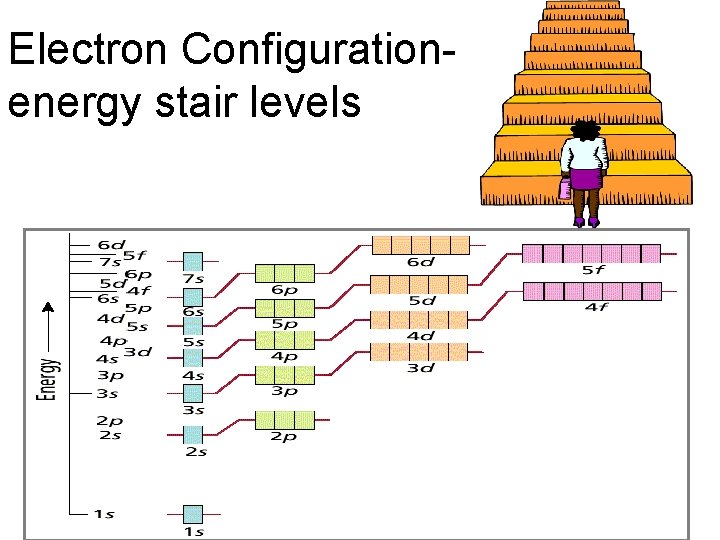
Electron Configuration- energy stair levels

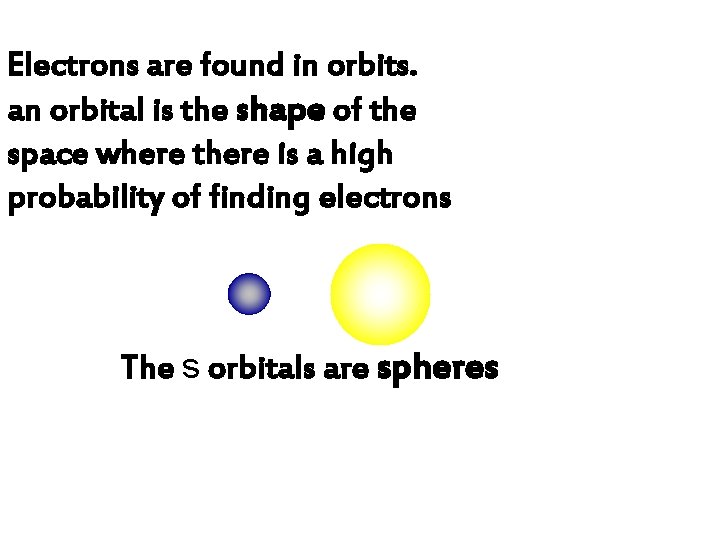
Electrons are found in orbits. an orbital is the shape of the space where there is a high probability of finding electrons The s orbitals are spheres

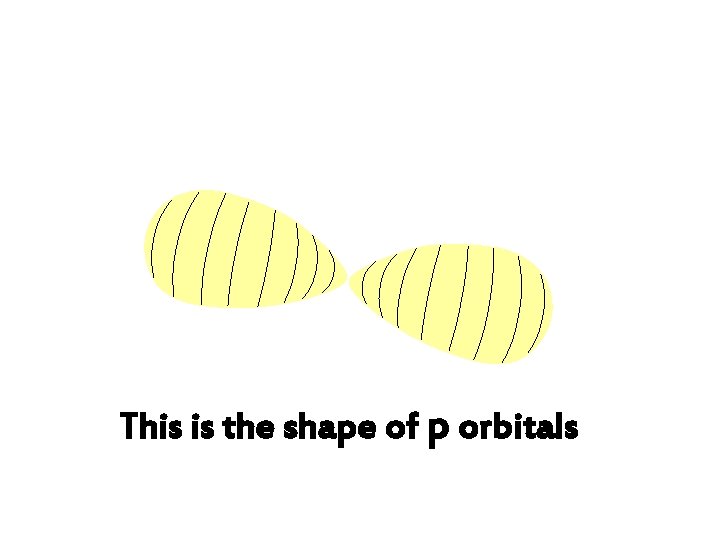
This is the shape of p orbitals

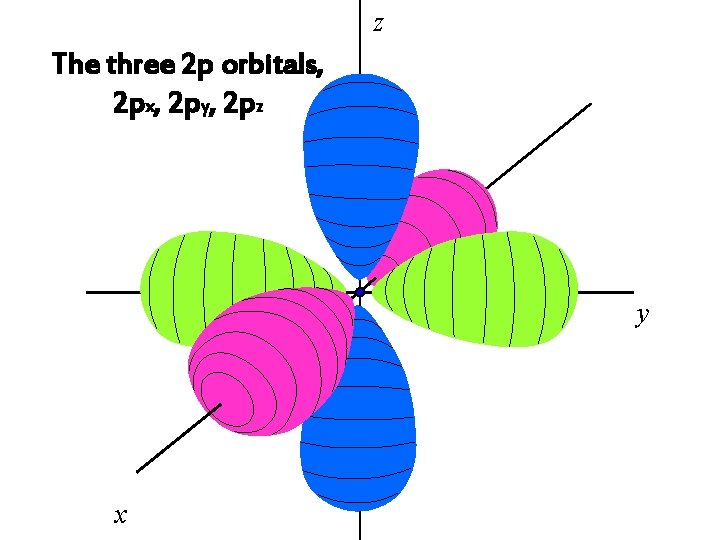
z The three 2 p orbitals, 2 px, 2 py, 2 pz y x

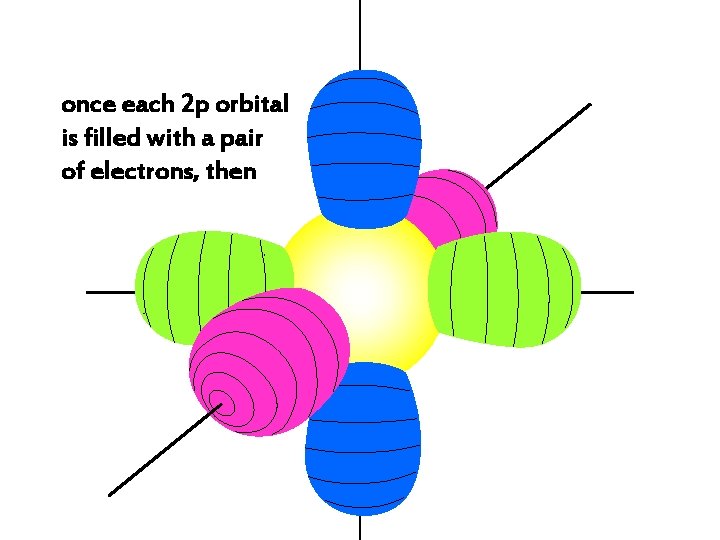
once each 2 p orbital is filled with a pair of electrons, then

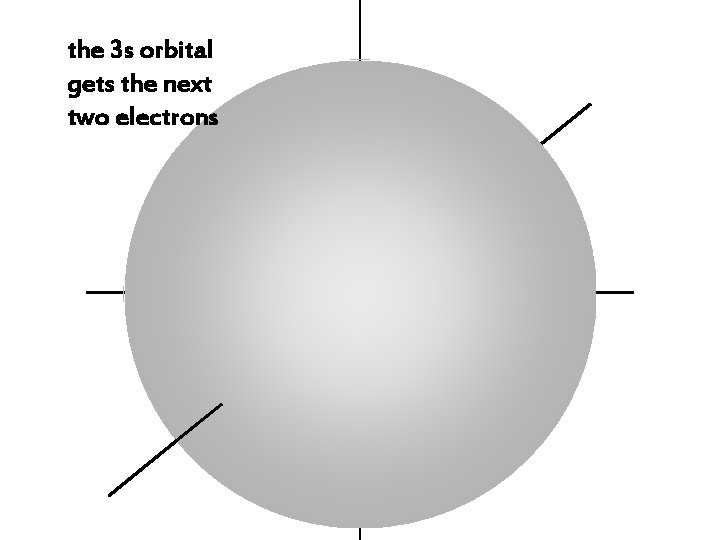
the 3 s orbital gets the next two electrons

the 3 s electrons have a higher energy than 1 s, 2 s, or 2 p electrons,

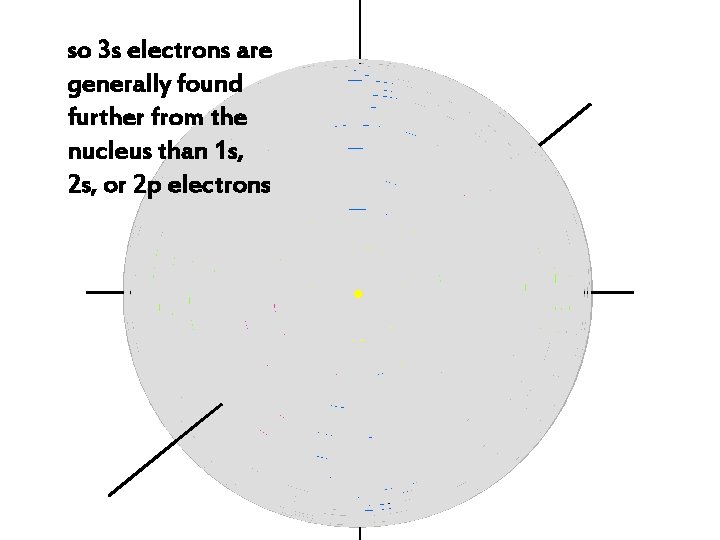
so 3 s electrons are generally found further from the nucleus than 1 s, 2 s, or 2 p electrons

Summary of Trend • Periodic Table and Periodic Trends • 1. Electron Configuration 3. Ionization Energy: Largest toward NE of PT 4. Electron Negativity: Most favorable NE of PT 2. Atomic Radius: Largest toward SW corner of PT

once each 2 p orbital is filled with a pair of electrons, then

Electron Configuration- energy stair levels

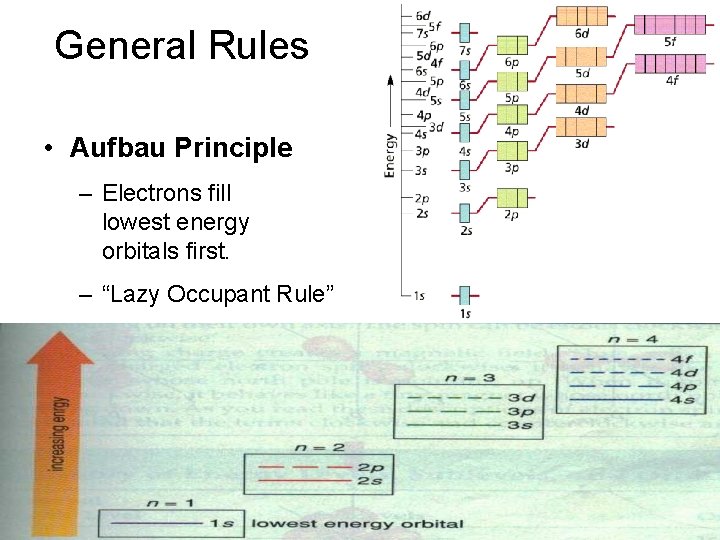
General Rules • Aufbau Principle – Electrons fill lowest energy orbitals first. – “Lazy Occupant Rule”

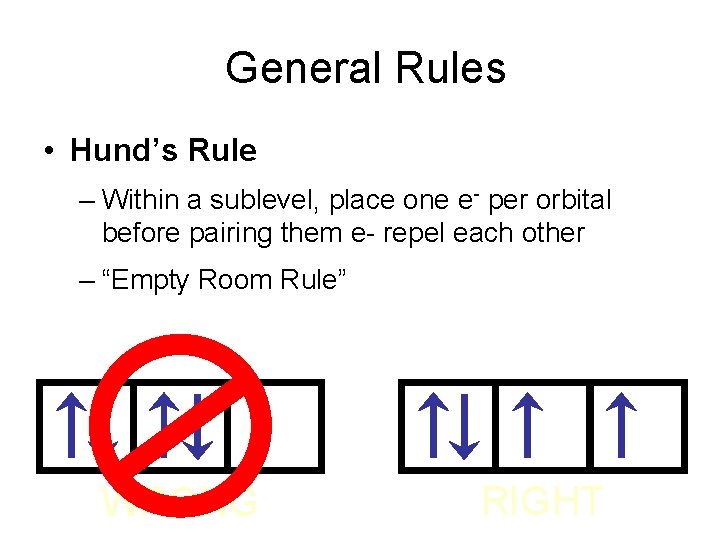
General Rules • Hund’s Rule – Within a sublevel, place one e- per orbital before pairing them e- repel each other – “Empty Room Rule” WRONG RIGHT

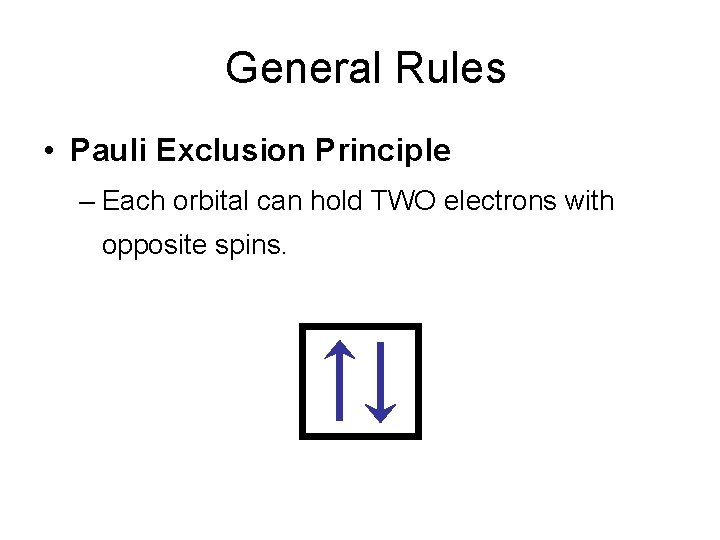
General Rules • Pauli Exclusion Principle – Each orbital can hold TWO electrons with opposite spins.

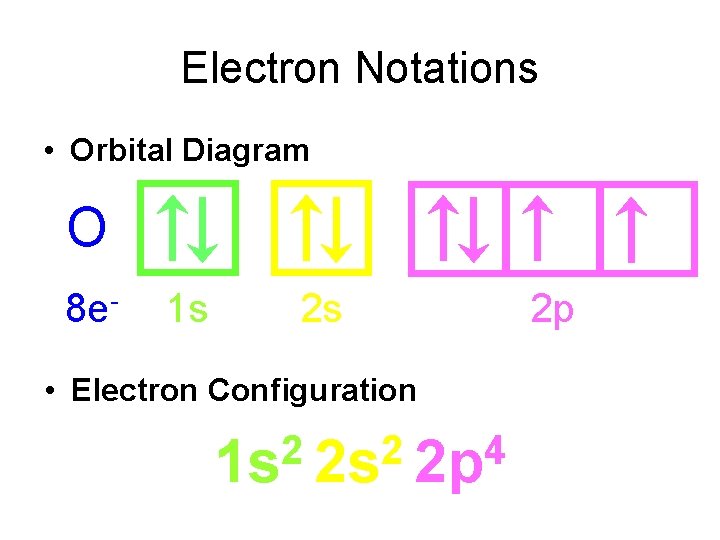
Electron Notations • Orbital Diagram O 8 e- 1 s 2 s • Electron Configuration 2 2 4 1 s 2 s 2 p 2 p

• Longhand e- Configuration S 16 e 6 2 2 2 1 s 2 s 2 p 3 s Core Electrons Valence Electrons • Shorthand e- Configuration S 16 e 4 3 p 2 4 [Ne] 3 s 3 p

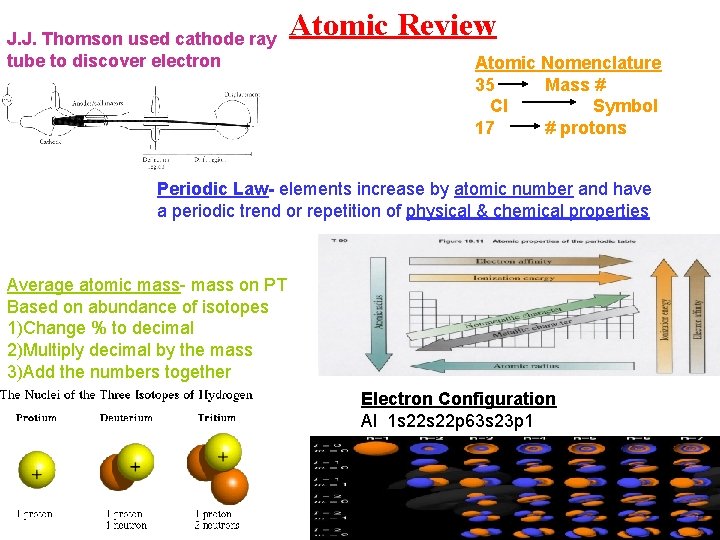
J. J. Thomson used cathode ray tube to discover electron Atomic Review Atomic Nomenclature 35 Mass # Cl Symbol 17 # protons Periodic Law- elements increase by atomic number and have a periodic trend or repetition of physical & chemical properties Average atomic mass- mass on PT Based on abundance of isotopes 1)Change % to decimal 2)Multiply decimal by the mass 3)Add the numbers together Electron Configuration Al 1 s 22 p 63 s 23 p 1

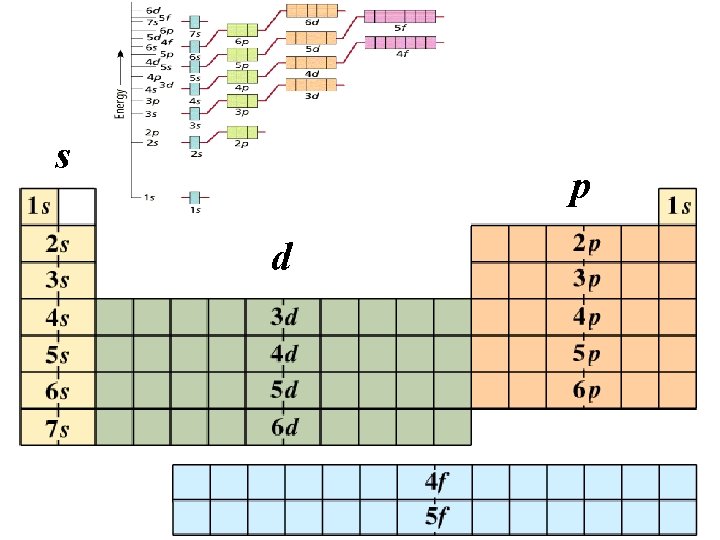
s p d © 1998 by Harcourt Brace & Company

e- configuration table 1 s 1 s 2 s 2 p 3 s 3 p 4 s 3 d 4 p 5 s 4 d 5 p 6 p 6 s La 5 d 7 s Ac 6 d 4 f 5 f

The Periodic Table s 1 1 2 3 4 5 6 7 s 2 d 1 d 2 d 3 d 4 d 5 d 6 d 7 d 8 d 9 d 10 p 1 p 2 p 3 p 4 p 5 p 6 1 2 H He 3 4 Li Be 11 12 Na Mg 19 20 5 6 7 8 B C N O F Ne 13 14 15 16 17 P S 33 34 Al Si 21 22 23 24 25 26 27 28 29 30 31 32 9 10 18 Cl Ar 35 36 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te 55 56 57 72 73 74 75 76 77 78 Cs Ba La Hf Ta W Re Os Ir 87 88 89 104 105 106 107 108 79 80 81 d p f 83 84 54 I Xe 85 86 Pt Au Hg Tl Pb Bi Po At Rn 109 110 111 114 116 118 Uuq Uuh Uuo 112 Fr Ra Ac Rf Db Sg Bh Hs Mt Uun Uuu Uub s 82 53 f 1 f 2 f 3 f 4 f 5 f 6 f 7 f 8 f 9 f 1 f 1 f 1 4 5 58 59 60 61 62 63 64 65 66 067 168 269 370 471 Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu 90 91 92 93 94 95 96 97 98 99 100 101 102 103 Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr



C. Periodic Patterns • Example - Hydrogen 1 1 s 1 st Period 1 st column of s-block

Electron Configuration • F • Cl • Al • Br

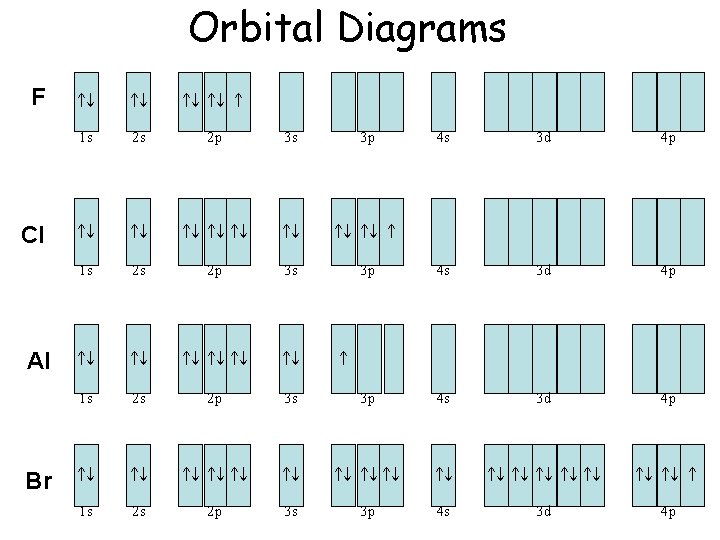
Orbital Diagrams F Cl Al Br 1 s 2 s 2 p 3 s 3 p 1 s 2 s 2 p 3 s 1 s 2 s 2 p 1 s 2 s 4 s 3 d 4 p 3 p 4 s 3 d 4 p 3 s 3 p 4 s 3 d 4 p 2 p 3 s 3 p 4 s 3 d 4 p

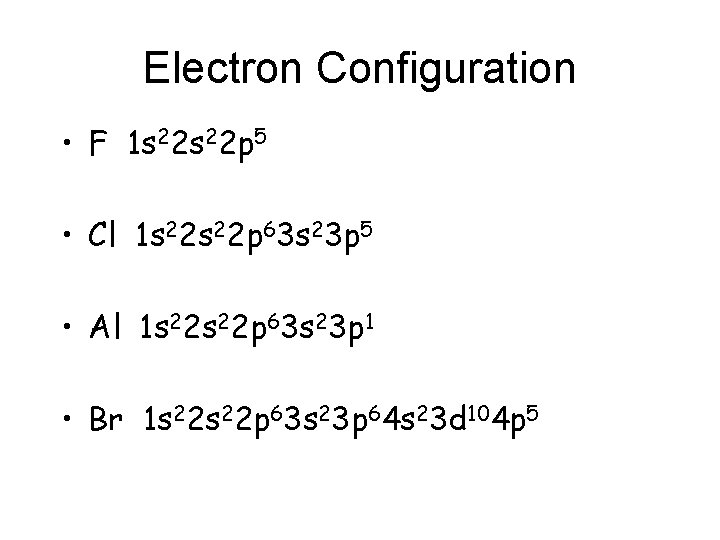
Electron Configuration • F 1 s 22 p 5 • Cl 1 s 22 p 63 s 23 p 5 • Al 1 s 22 p 63 s 23 p 1 • Br 1 s 22 p 63 s 23 p 64 s 23 d 104 p 5

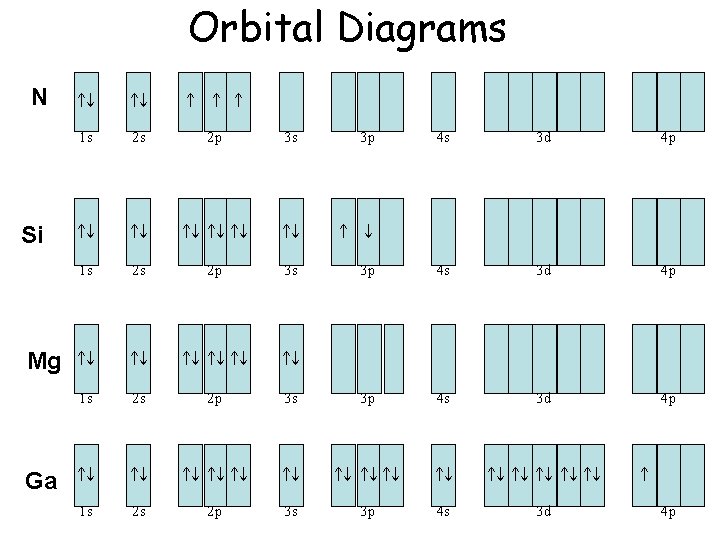
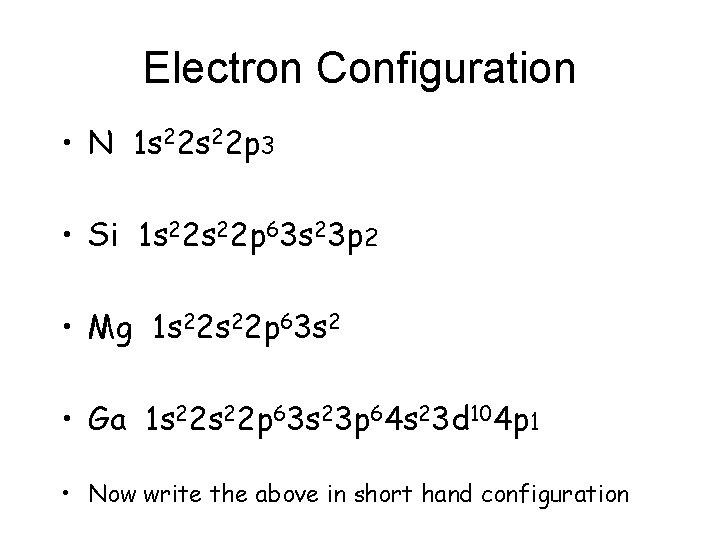
Write Orbital and Electron Configurations • N • Si • Mg • Ga

Orbital Diagrams N Si Mg Ga 1 s 2 s 2 p 3 s 1 s 2 s 2 p 1 s 2 s 3 p 4 s 3 d 4 p 3 s 3 p 4 s 3 d 4 p 2 p 3 s 3 p 4 s 3 d 4 p

Electron Configuration • N 1 s 22 p 3 • Si 1 s 22 p 63 s 23 p 2 • Mg 1 s 22 p 63 s 2 • Ga 1 s 22 p 63 s 23 p 64 s 23 d 104 p 1 • Now write the above in short hand configuration
![Electron Configuration N He2 s 22 p 3 Electron Configuration • N [He]2 s 22 p 3](https://slidetodoc.com/presentation_image_h/265ce1b76b2fa1010934e28daa8bdc65/image-114.jpg)
Electron Configuration • N [He]2 s 22 p 3
![Electron Configuration Si Ne3 s 23 p 2 Electron Configuration • Si [Ne]3 s 23 p 2](https://slidetodoc.com/presentation_image_h/265ce1b76b2fa1010934e28daa8bdc65/image-115.jpg)
Electron Configuration • Si [Ne]3 s 23 p 2
![Electron Configuration Mg Ne3 s 2 Electron Configuration • Mg [Ne]3 s 2](https://slidetodoc.com/presentation_image_h/265ce1b76b2fa1010934e28daa8bdc65/image-116.jpg)
Electron Configuration • Mg [Ne]3 s 2
![Electron Configuration Ga Ar4 s 23 d 104 p 1 Electron Configuration • Ga [Ar]4 s 23 d 104 p 1](https://slidetodoc.com/presentation_image_h/265ce1b76b2fa1010934e28daa8bdc65/image-117.jpg)
Electron Configuration • Ga [Ar]4 s 23 d 104 p 1
![Periodic Patterns Example Germanium Ar 2 4 s 10 3 d Periodic Patterns • Example - Germanium [Ar] 2 4 s 10 3 d](https://slidetodoc.com/presentation_image_h/265ce1b76b2fa1010934e28daa8bdc65/image-118.jpg)
Periodic Patterns • Example - Germanium [Ar] 2 4 s 10 3 d 2 4 p

• Notable families of the Periodic Table Halogen Noble Gas Chalcogens Alkaline (earth) Transition Metals

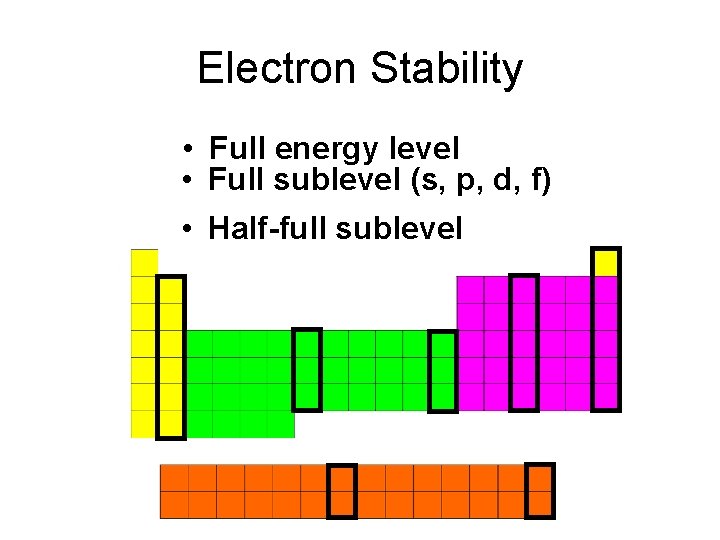
Electron Stability • Full energy level • Full sublevel (s, p, d, f) • Half-full sublevel
![Stability Electron Configuration Exceptions Copper EXPECT ACTUALLY Ar 4 s 2 3 Stability • Electron Configuration Exceptions – Copper EXPECT: ACTUALLY: [Ar] 4 s 2 3](https://slidetodoc.com/presentation_image_h/265ce1b76b2fa1010934e28daa8bdc65/image-121.jpg)
Stability • Electron Configuration Exceptions – Copper EXPECT: ACTUALLY: [Ar] 4 s 2 3 d 9 [Ar] 4 s 1 3 d 10 – Copper gains stability with a full d-sublevel.

“ 5 Facts 5 Pictures” Review Interesting Fact 1 - define or explain Interesting Fact 3 define or explain [picture] [Picture] Most Important Concept or Fact 5 Describe and explain What you learned [Picture] Interesting Fact 2 - define or explain [picture] Interesting Fact 4 - define or explain [picture]

J. J. Thomson used cathode ray tube to discover electron Atomic Review Atomic Nomenclature 35 Mass # Cl Symbol 17 # protons Periodic Law- elements increase by atomic number and have a periodic trend or repetition of physical & chemical properties Average atomic mass- mass on PT Based on abundance of isotopes 1)Change % to decimal 2)Multiply decimal by the mass 3)Add the numbers together Electron Configuration Al 1 s 22 p 63 s 23 p 1
![D Stability Electron Configuration Exceptions Chromium EXPECT ACTUALLY Ar 4 s 2 D. Stability • Electron Configuration Exceptions – Chromium EXPECT: ACTUALLY: [Ar] 4 s 2](https://slidetodoc.com/presentation_image_h/265ce1b76b2fa1010934e28daa8bdc65/image-124.jpg)
D. Stability • Electron Configuration Exceptions – Chromium EXPECT: ACTUALLY: [Ar] 4 s 2 3 d 4 [Ar] 4 s 1 3 d 5 – Chromium gains stability with a half-full dsublevel.

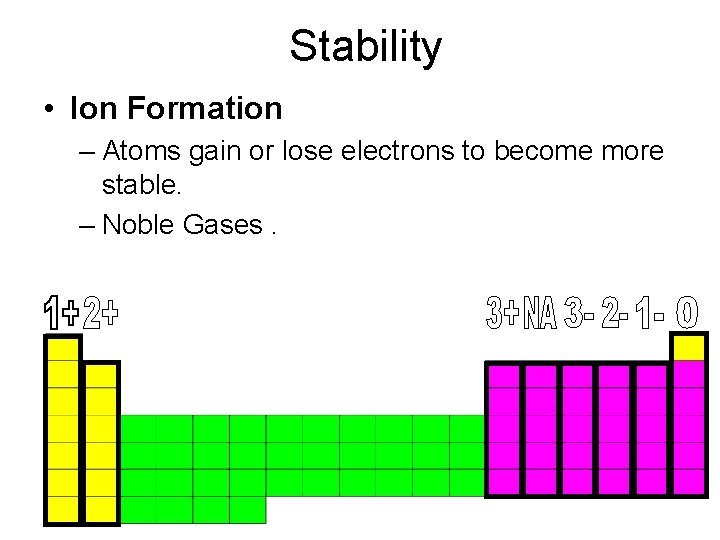
Stability • Ion Formation – Atoms gain or lose electrons to become more stable. – Noble Gases.

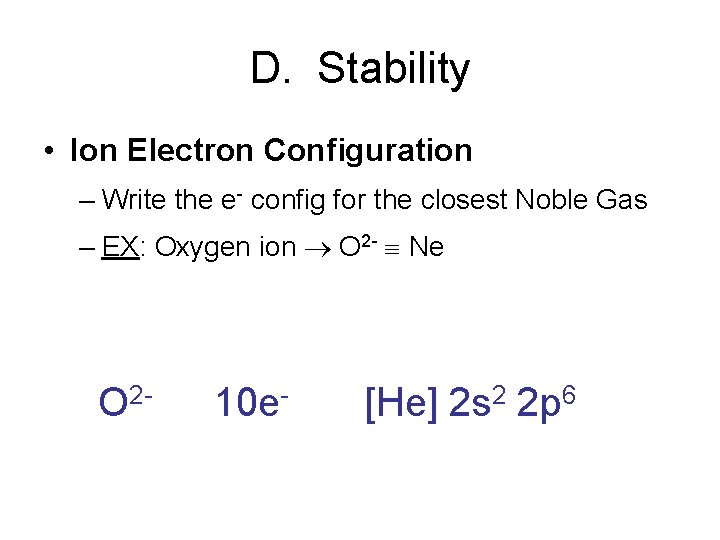
D. Stability • Ion Electron Configuration – Write the e- config for the closest Noble Gas – EX: Oxygen ion O 2 - Ne O 2 - 10 e- [He] 2 s 2 2 p 6

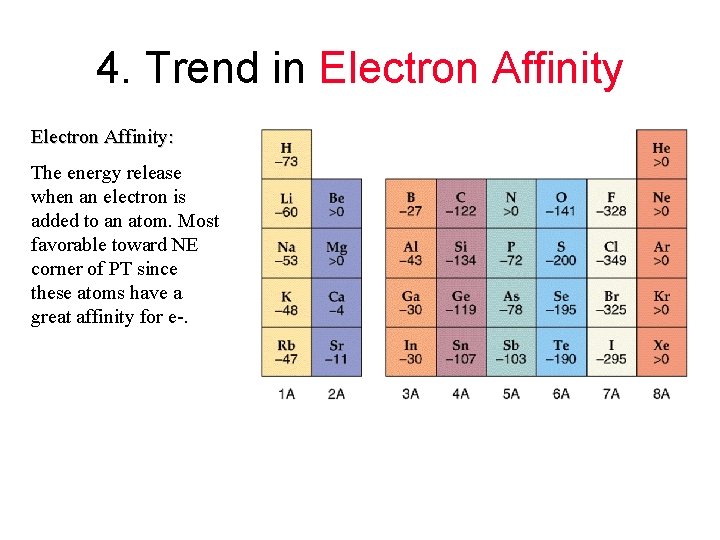
4. Trend in Electron Affinity: The energy release when an electron is added to an atom. Most favorable toward NE corner of PT since these atoms have a great affinity for e-.

Periods- left to right Groups- up & down, numbered 1 -18 or 1 A-8 A, Representative Elements- Group A Elements, Group 1, 2, & 13 -18 (8 groups represented)
 Atom models history
Atom models history History of atom discovery
History of atom discovery The structure of the atom section 2 defining the atom
The structure of the atom section 2 defining the atom Kelemahan atom thomson
Kelemahan atom thomson Lesson 11 atomic pudding models of the atom
Lesson 11 atomic pudding models of the atom Aristotle atomic
Aristotle atomic Lesson 11 atomic pudding models of the atom answer key
Lesson 11 atomic pudding models of the atom answer key Modals and semimodals
Modals and semimodals Early cpr and early defibrillation can: *
Early cpr and early defibrillation can: * Atom economy green chemistry
Atom economy green chemistry Functional groups ib chemistry
Functional groups ib chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Atomic history webquest
Atomic history webquest Democritus atomic model
Democritus atomic model History of the atom john dalton
History of the atom john dalton Demokritos atom modeli
Demokritos atom modeli History of the atom graphic organizer
History of the atom graphic organizer Plum pudding model
Plum pudding model Section 17-2 earth's early history answer key
Section 17-2 earth's early history answer key 17-2 earth's early history
17-2 earth's early history Section 17-2 earth's early history
Section 17-2 earth's early history Egypt floral design
Egypt floral design Early beginnings of tourism
Early beginnings of tourism Egyptian floral design
Egyptian floral design What do historians call the early period of human history
What do historians call the early period of human history Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Slidetodoc
Slidetodoc Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Tư thế worm breton
Tư thế worm breton Chúa yêu trần thế
Chúa yêu trần thế Các môn thể thao bắt đầu bằng tiếng bóng
Các môn thể thao bắt đầu bằng tiếng bóng Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính thế năng
Công thức tính thế năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 101012 bằng
101012 bằng Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Bàn tay mà dây bẩn
Bàn tay mà dây bẩn Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Ví dụ giọng cùng tên
Ví dụ giọng cùng tên Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Phối cảnh
Phối cảnh Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Dạng đột biến một nhiễm là
Dạng đột biến một nhiễm là So nguyen to
So nguyen to Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Sự nuôi và dạy con của hươu
Sự nuôi và dạy con của hươu Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Sơ đồ cơ thể người
Sơ đồ cơ thể người Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Also history physical
Also history physical Etilbenzen + cl2
Etilbenzen + cl2 Dalton atom modeli
Dalton atom modeli Fiserove formule
Fiserove formule Boron atom
Boron atom Recall what an atom is.
Recall what an atom is. Atom subatomic particles
Atom subatomic particles Which equation represents sublimation?
Which equation represents sublimation? Atom learning contact number
Atom learning contact number What is quantum mechanical model
What is quantum mechanical model Levels of organization atom
Levels of organization atom Democritus atomic model diagram
Democritus atomic model diagram Atom of halogen
Atom of halogen Whats nucleus
Whats nucleus The atom: from philosophical idea to scientific theory
The atom: from philosophical idea to scientific theory Atoms are very small
Atoms are very small Bunyi teori dalton
Bunyi teori dalton Lambang atom no 31
Lambang atom no 31 Nyatakan dalam mol dari 3 01 x 1022 atom besi
Nyatakan dalam mol dari 3 01 x 1022 atom besi Aleek atom
Aleek atom Elik atom
Elik atom Stereoizomerija
Stereoizomerija Centrum chiralności
Centrum chiralności Solving schrodinger equation for hydrogen atom
Solving schrodinger equation for hydrogen atom Hukum triade dobereiner
Hukum triade dobereiner Jari jari atom pada sistem periodik
Jari jari atom pada sistem periodik Lambang atom nomor 31
Lambang atom nomor 31 Atom senyawa
Atom senyawa Keunikan atom karbon
Keunikan atom karbon Kime göre ben neyim tekniği
Kime göre ben neyim tekniği Schrodinger wave mechanical model
Schrodinger wave mechanical model Atom l
Atom l Drawing atoms practice
Drawing atoms practice Lambang atom no 31
Lambang atom no 31 Ionic radii trends
Ionic radii trends Peta konsep fisika inti
Peta konsep fisika inti Labeled parts of an atom
Labeled parts of an atom Jari jari atom
Jari jari atom Axz atom
Axz atom Can an atom have more neutrons than protons
Can an atom have more neutrons than protons Erwin schrödinger model of atom
Erwin schrödinger model of atom Gcc
Gcc Atom numarası
Atom numarası Nomor atom nomor massa
Nomor atom nomor massa Tentukan jumlah atom cp cs ct ck
Tentukan jumlah atom cp cs ct ck Phet build atom
Phet build atom Lewis structures vsepr
Lewis structures vsepr Cell atom molecule
Cell atom molecule Lithium oxide ionic bond
Lithium oxide ionic bond Elik atom
Elik atom Isi teori niels bohr
Isi teori niels bohr When was the billiard ball model discovered
When was the billiard ball model discovered Number of neutron gold
Number of neutron gold Calcium atom bohr model
Calcium atom bohr model Dot
Dot Green chemistry
Green chemistry Atom in greek
Atom in greek Susunan atom klorin
Susunan atom klorin Zeemanning
Zeemanning Peta konsep fisika inti dan radioaktivitas
Peta konsep fisika inti dan radioaktivitas Atom economy formula
Atom economy formula Electronegativity is the ability of an atom to
Electronegativity is the ability of an atom to