History of Benzene History of Benzene 1824 1825

History of Benzene

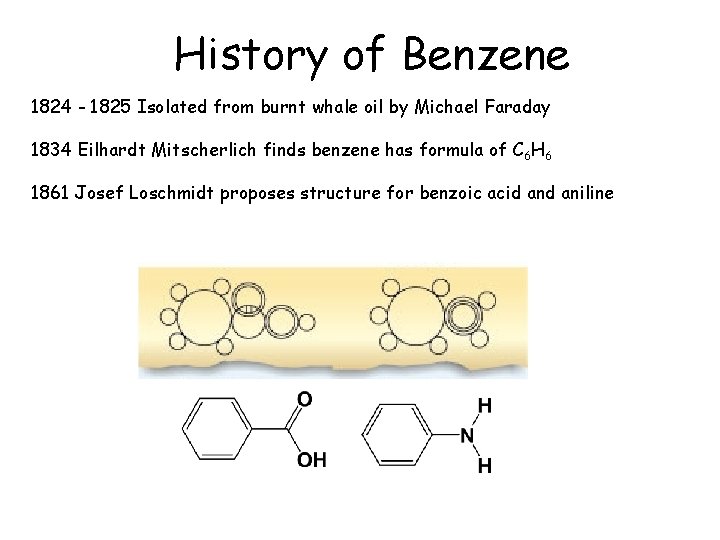
History of Benzene 1824 - 1825 Isolated from burnt whale oil by Michael Faraday 1834 Eilhardt Mitscherlich finds benzene has formula of C 6 H 6

History of Benzene 1824 - 1825 Isolated from burnt whale oil by Michael Faraday 1834 Eilhardt Mitscherlich finds benzene has formula of C 6 H 6 1861 Josef Loschmidt proposes structure for benzoic acid aniline
History of Benzene 1824 - 1825 Isolated from burnt whale oil by Michael Faraday 1834 Eilhardt Mitscherlich finds benzene has formula of C 6 H 6 1861 Josef Loschmidt proposes structure for benzoic acid aniline

History of Benzene 1865 Friedrich August Kekulé steps into the picture

History of Benzene 1865 Kekulé has a dream
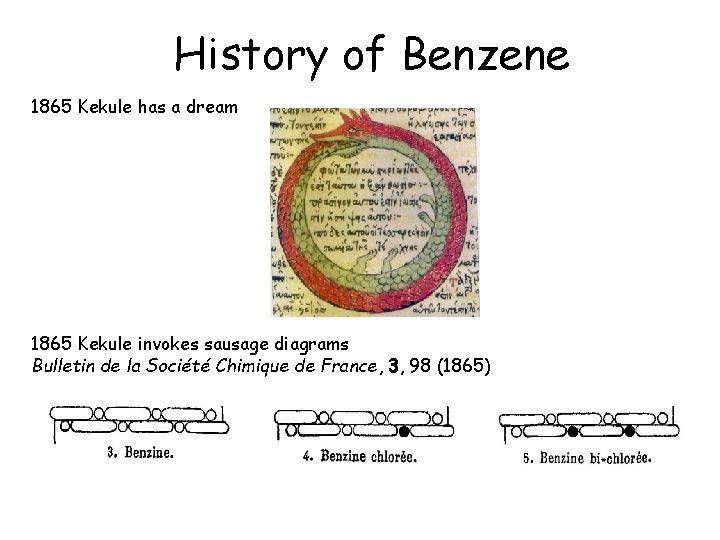
History of Benzene 1865 Kekule has a dream 1865 Kekule invokes sausage diagrams Bulletin de la Société Chimique de France, 3, 98 (1865)
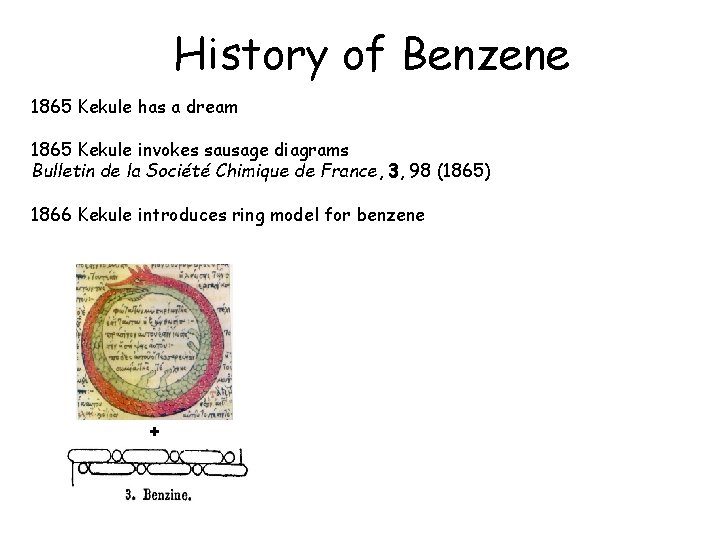
History of Benzene 1865 Kekule has a dream 1865 Kekule invokes sausage diagrams Bulletin de la Société Chimique de France, 3, 98 (1865) 1866 Kekule introduces ring model for benzene +
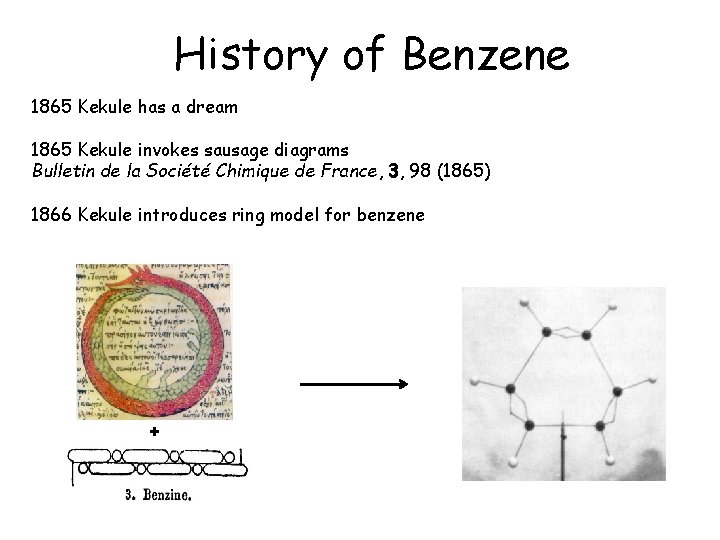
History of Benzene 1865 Kekule has a dream 1865 Kekule invokes sausage diagrams Bulletin de la Société Chimique de France, 3, 98 (1865) 1866 Kekule introduces ring model for benzene +
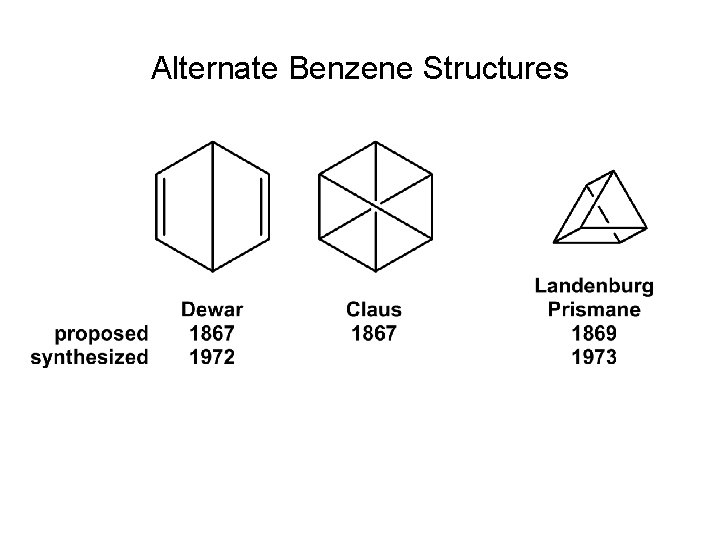
Alternate Benzene Structures

Hückel’s Rules for aromaticity 1. 2 3. 4.
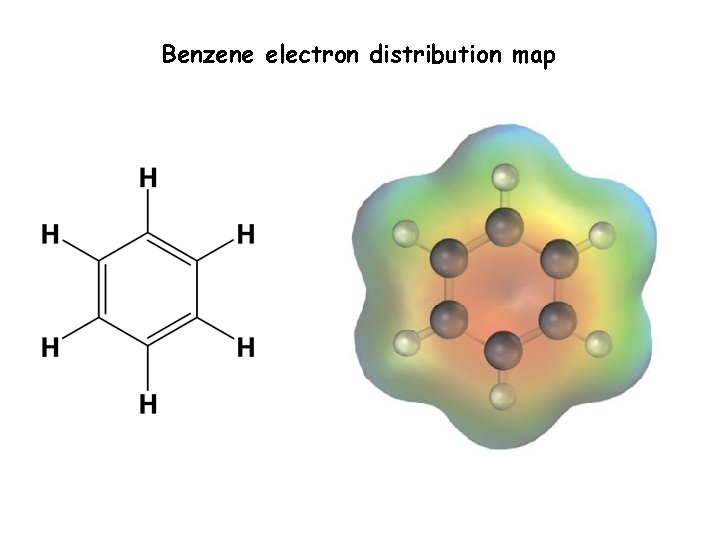
Benzene electron distribution map

Hückel’s Rules for aromaticity Aromaticity vs. antiaromaticity
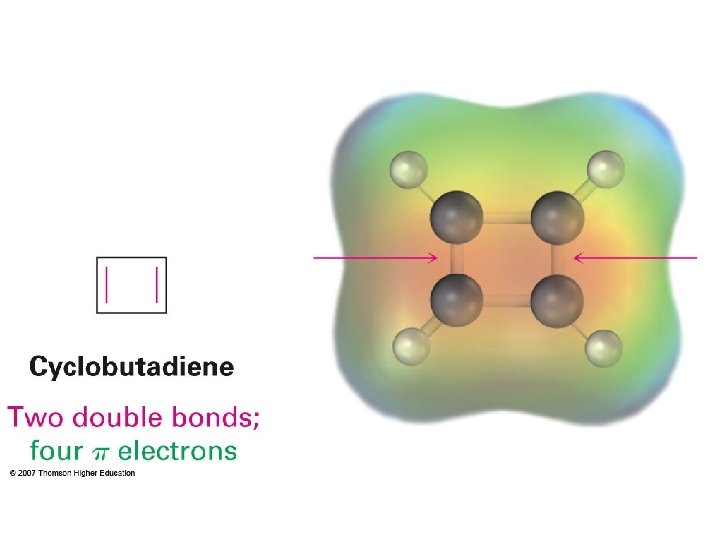
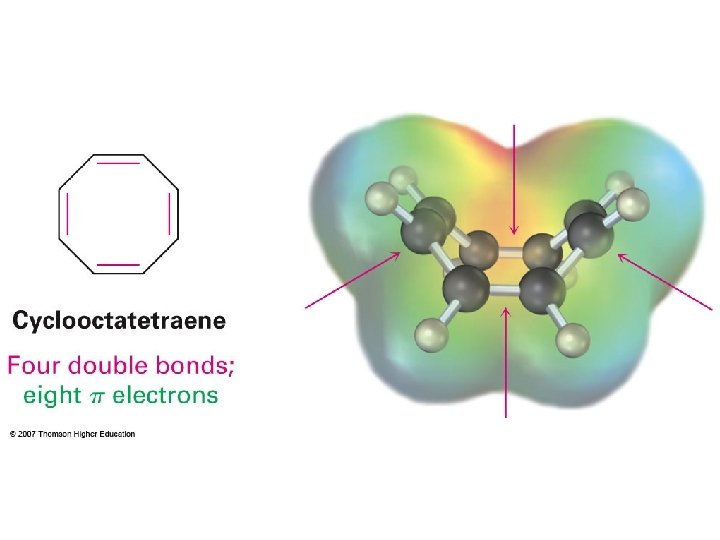

Hückel’s Rules for aromaticity Aromaticity vs. antiaromaticity Why 4 n + 2 for aromatic and 4 n for antiaromatic? Frost’s cycle
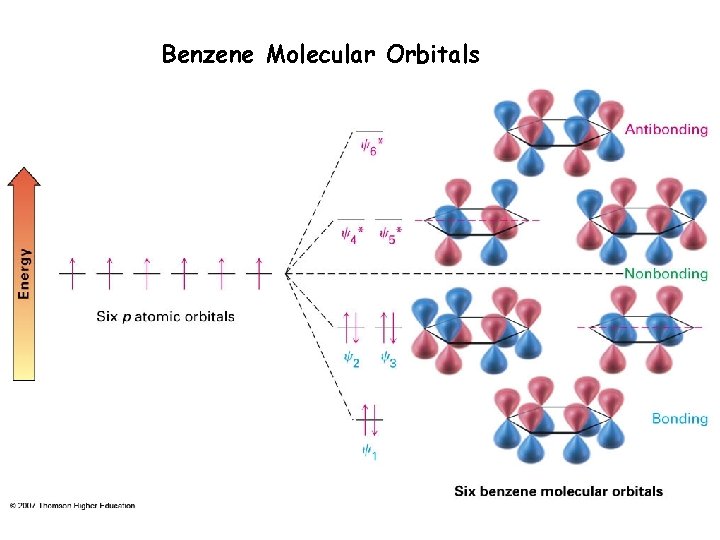
Benzene Molecular Orbitals

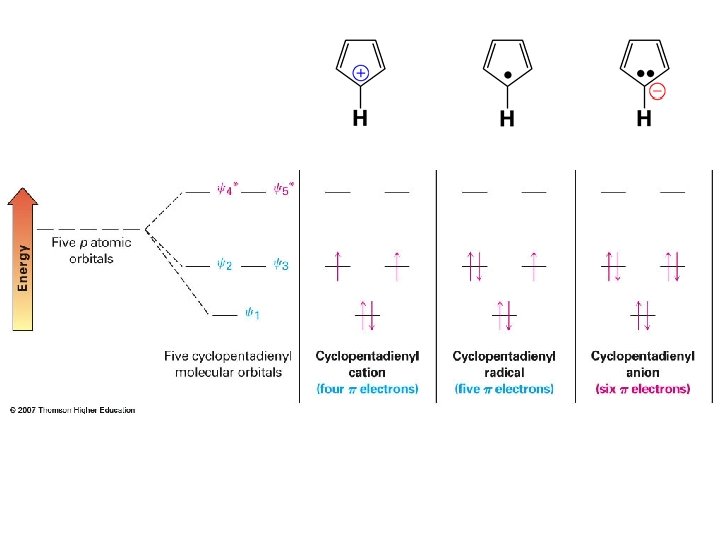
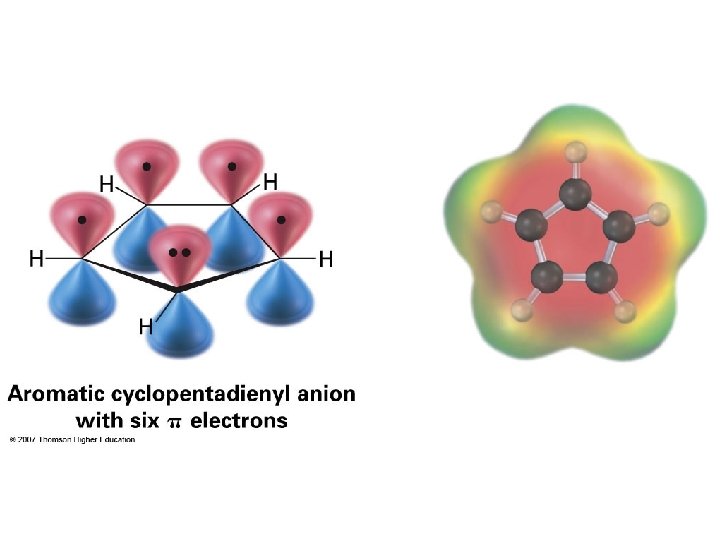
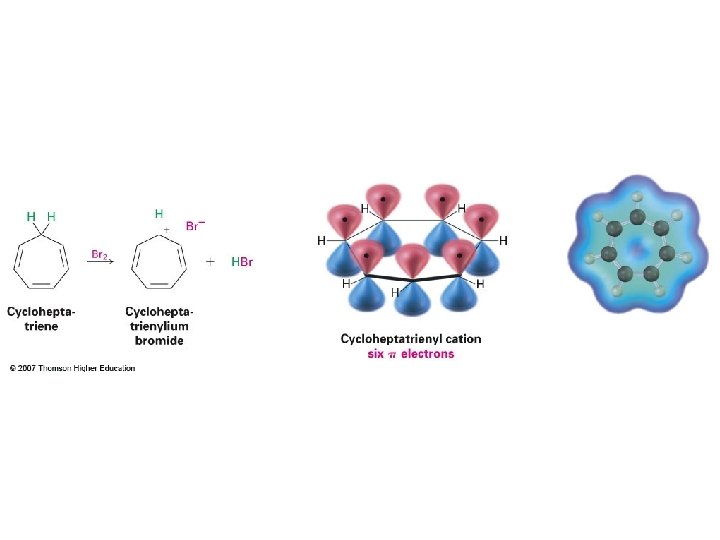
Cyclopentadienyl ions
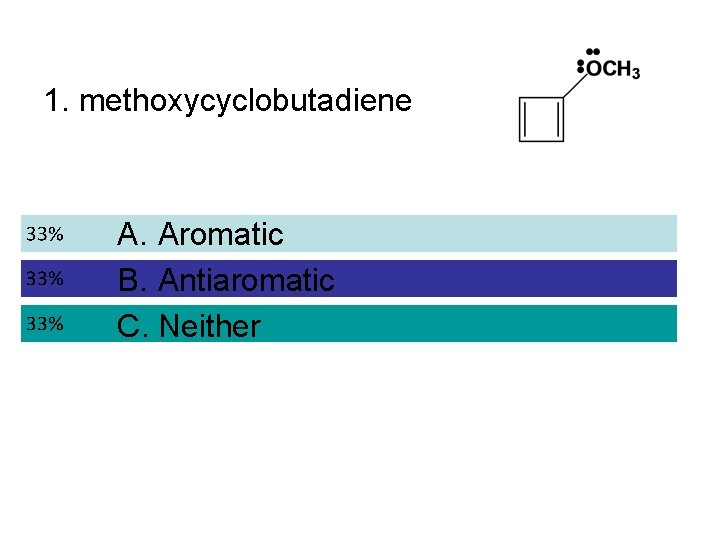
1. methoxycyclobutadiene A. Aromatic B. Antiaromatic C. Neither
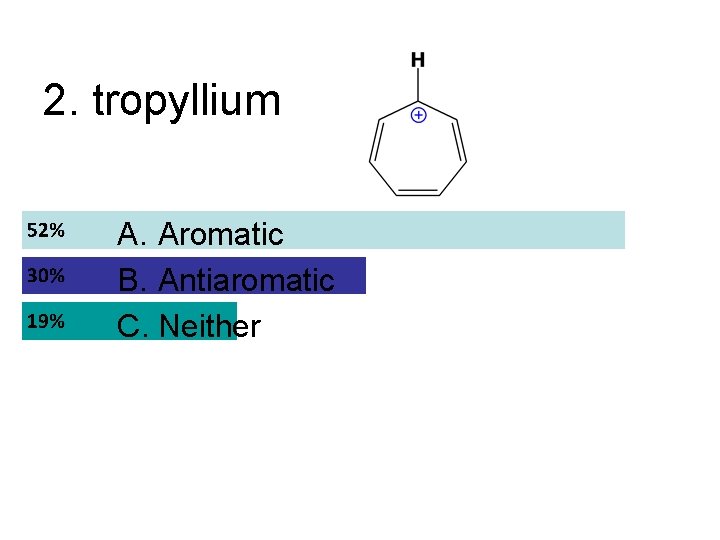
2. tropyllium A. Aromatic B. Antiaromatic C. Neither
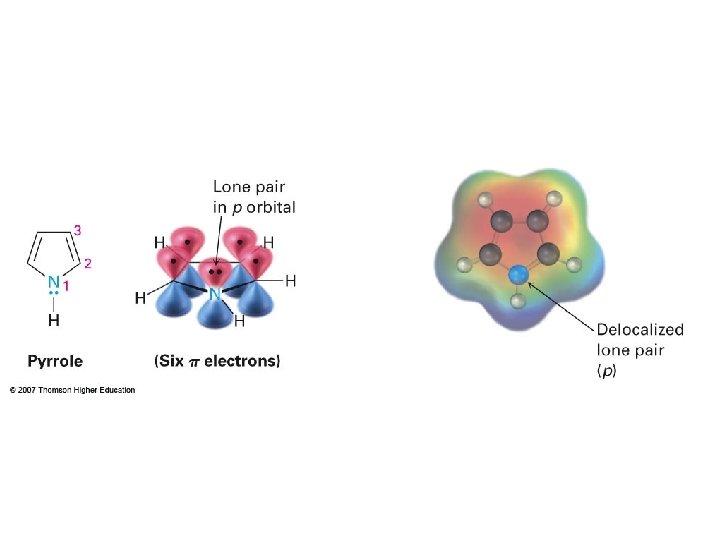
3. pyrrole A. Aromatic B. Antiaromatic C. Neither
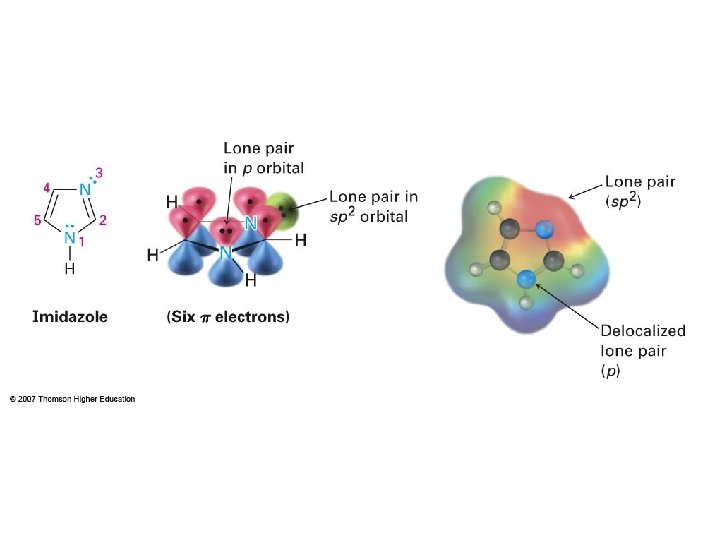
4. imidazole A. Aromatic B. Antiaromatic C. Neither
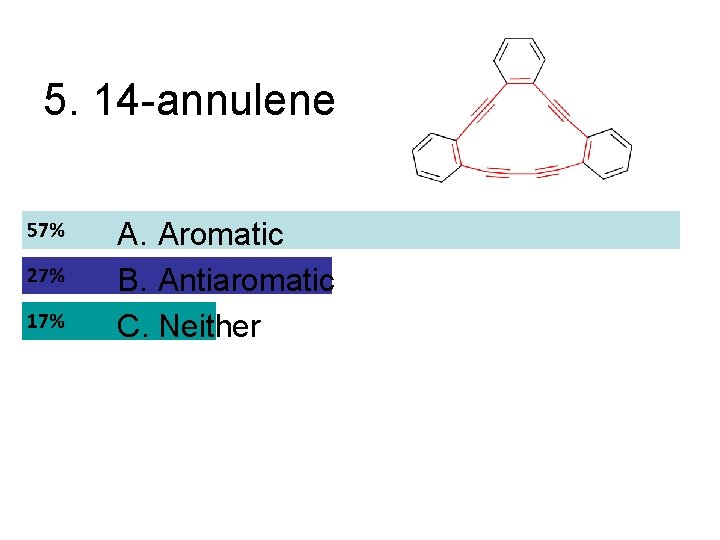
5. 14 -annulene A. Aromatic B. Antiaromatic C. Neither
x x
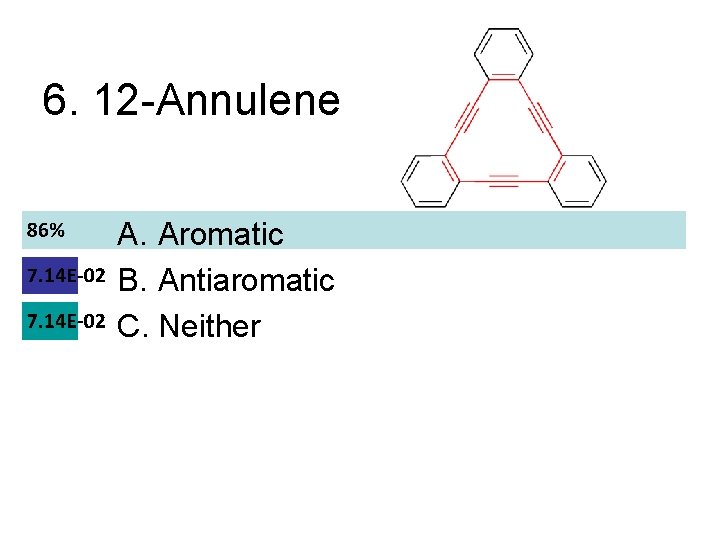
6. 12 -Annulene A. Aromatic B. Antiaromatic C. Neither
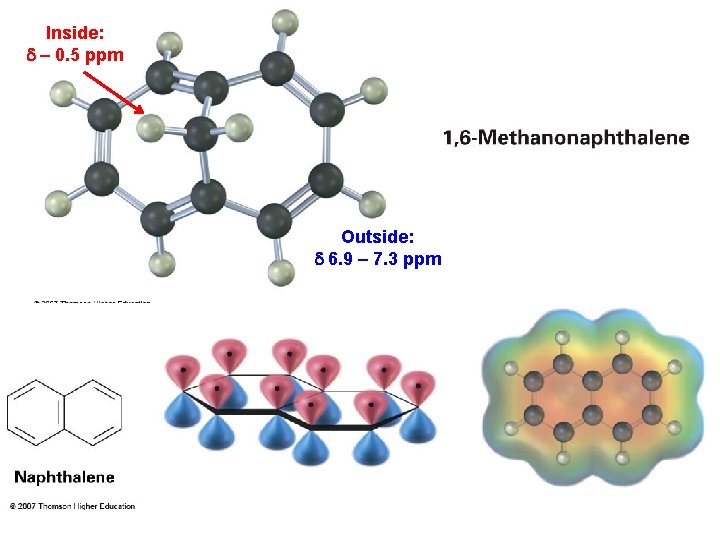
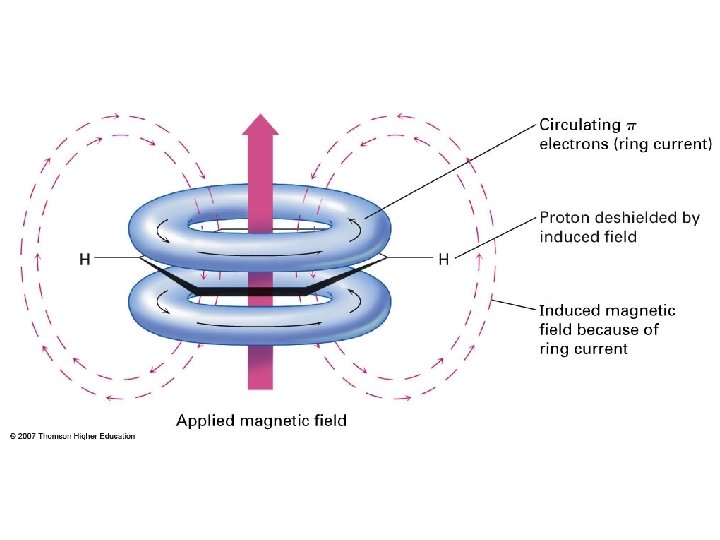
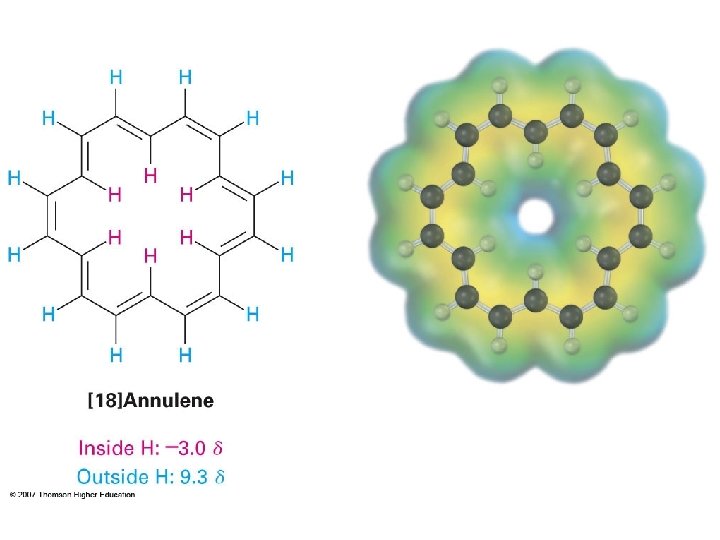
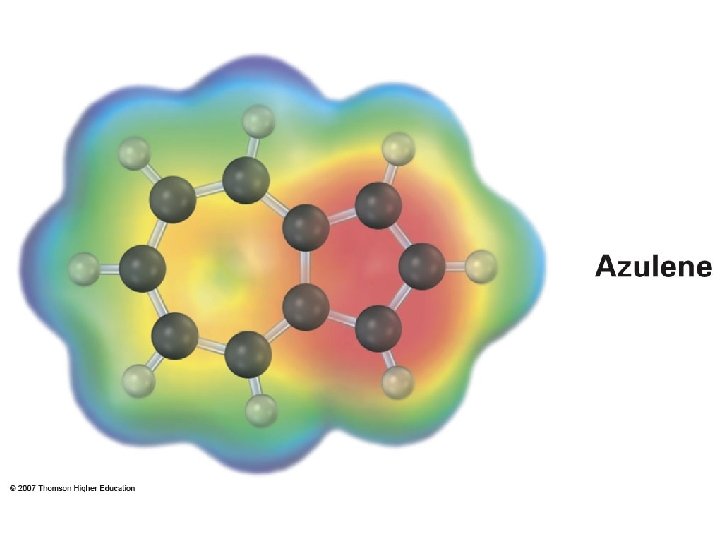
Inside: d – 0. 5 ppm Outside: d 6. 9 – 7. 3 ppm

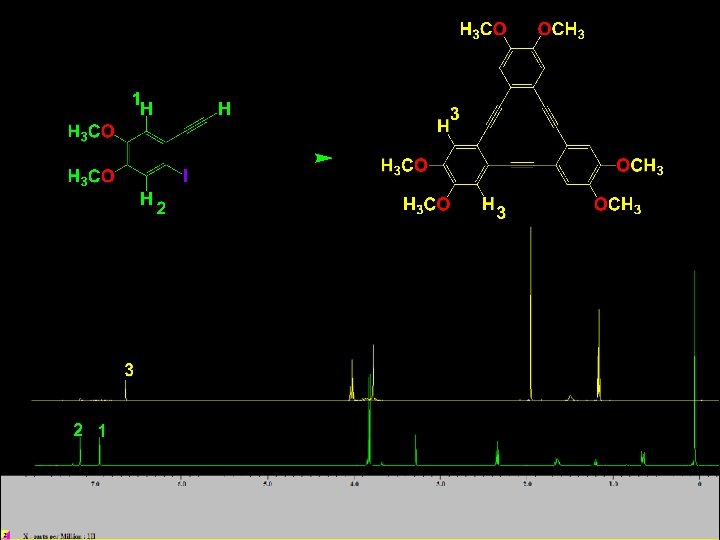
CHE 311 Starts Here Section 9. 6
Reactivity of Benzene Electrophilic Aromatic Substitution
Reactivity of Benzene Electrophilic Aromatic Substitution The Mechanism
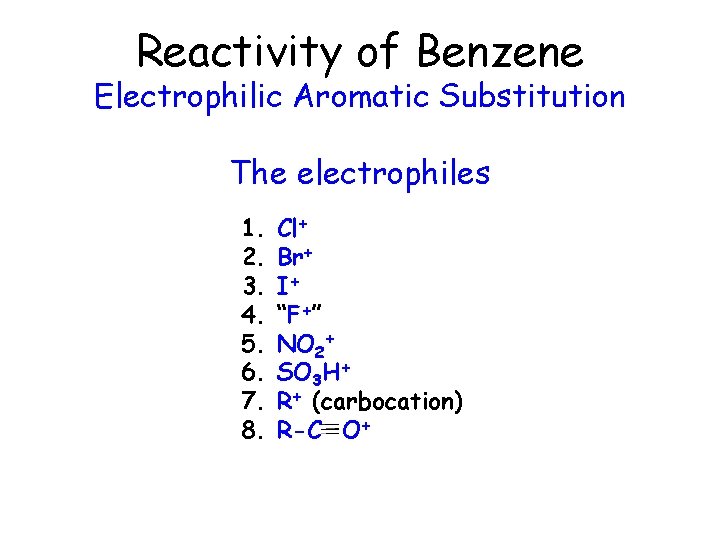
Reactivity of Benzene Electrophilic Aromatic Substitution The electrophiles 1. 2. 3. 4. 5. 6. 7. 8. Cl+ Br+ I+ “F+” NO 2+ SO 3 H+ R+ (carbocation) R-C O+
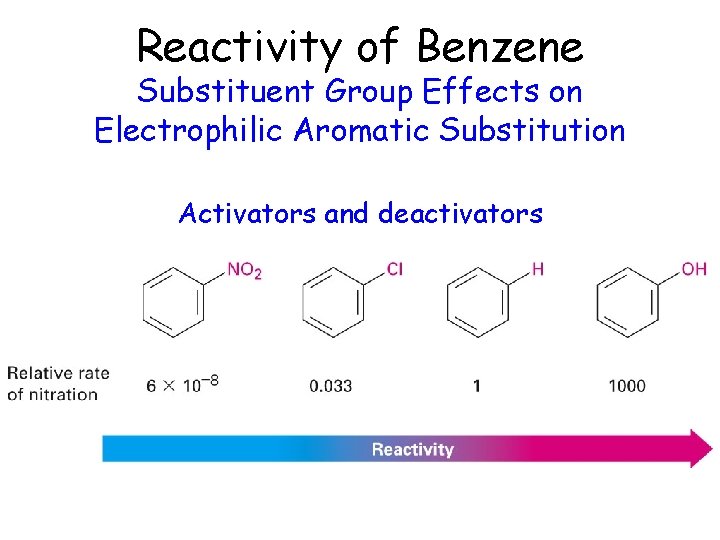
Reactivity of Benzene Substituent Group Effects on Electrophilic Aromatic Substitution Activators and deactivators
Reactivity of Benzene Substituent Group Effects on Electrophilic Aromatic Substitution

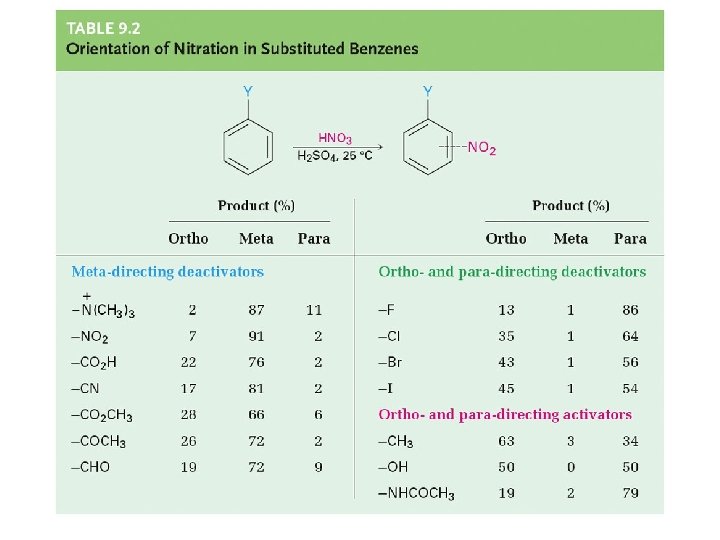
Reactivity of Benzene Substituent Group Effects on Electrophilic Aromatic Substitution Directing Group Effects
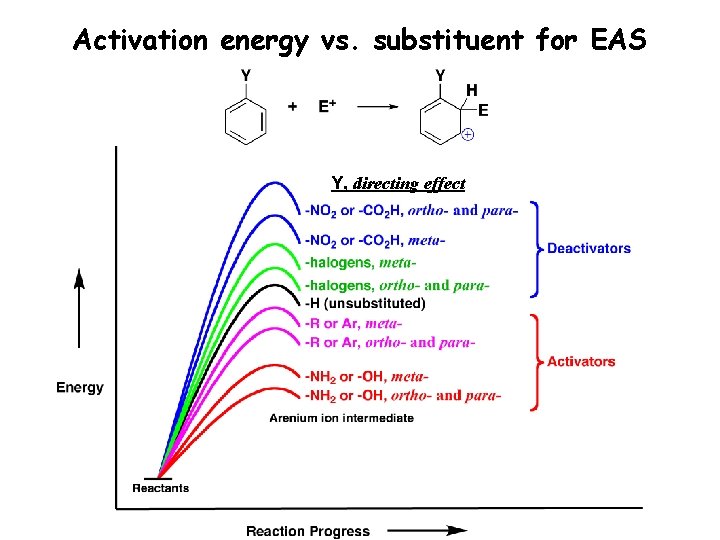
Activation energy vs. substituent for EAS Y, directing effect
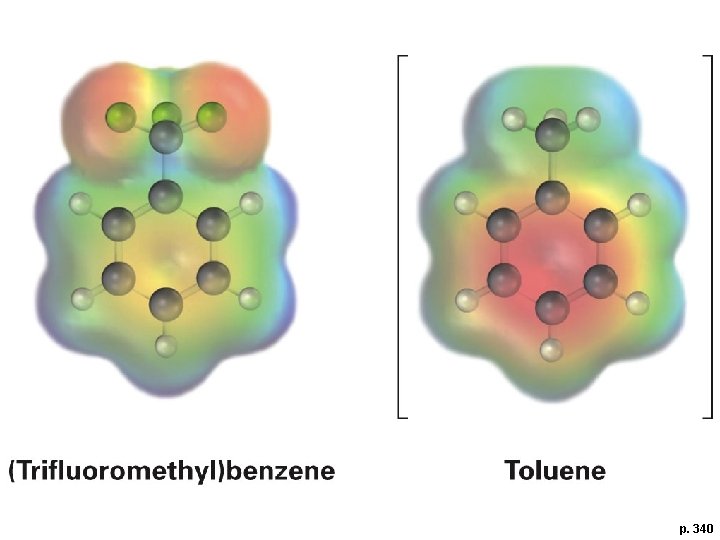
p. 340
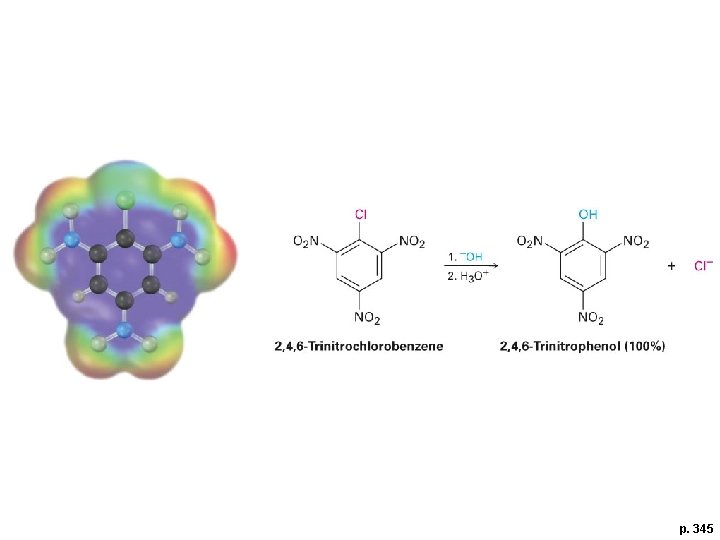
p. 345
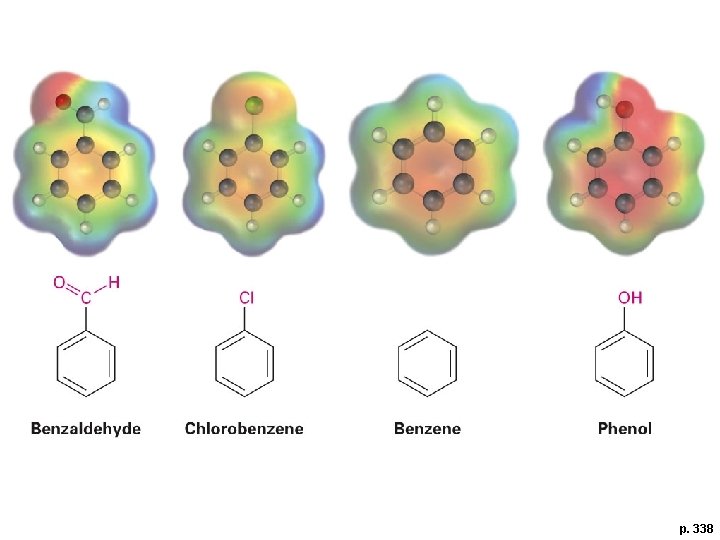
p. 338
Aromatic Biomolecules 1. Amino acids 2. Nucleic acids
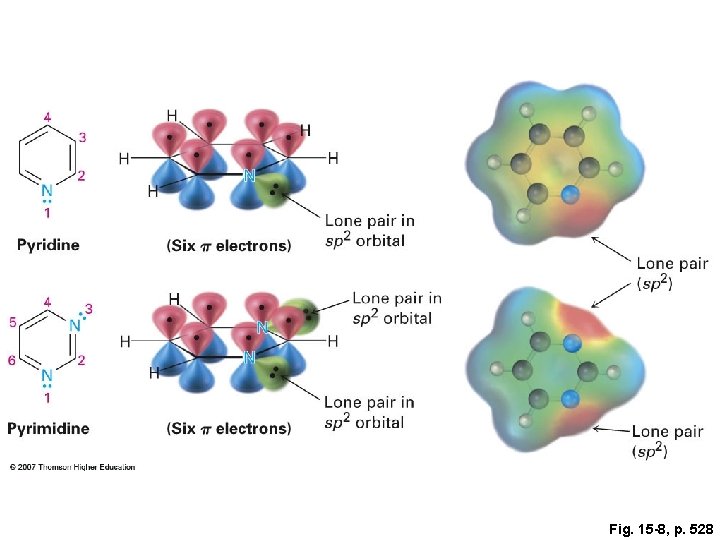
Fig. 15 -8, p. 528
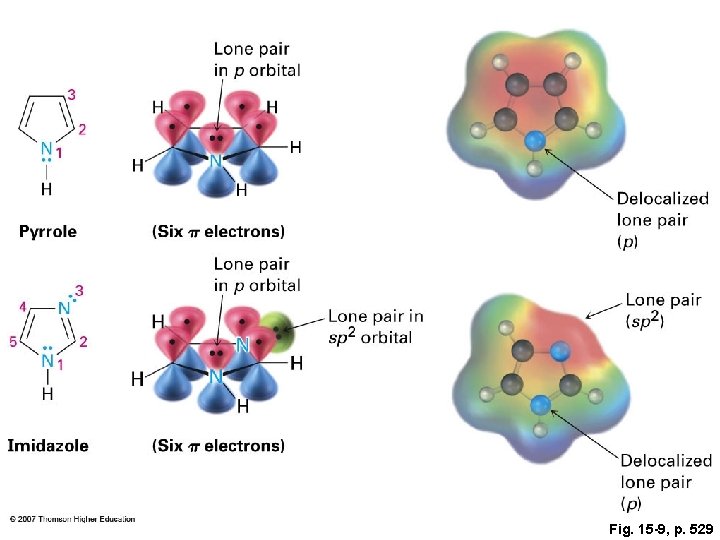
Fig. 15 -9, p. 529
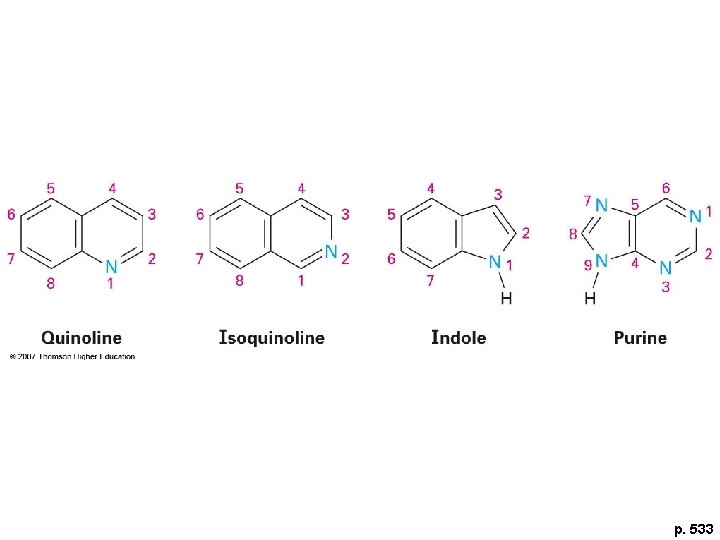
p. 533
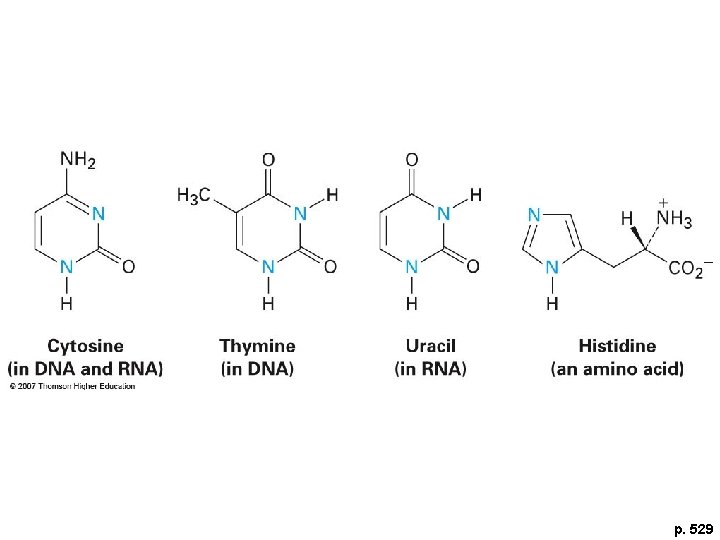
p. 529
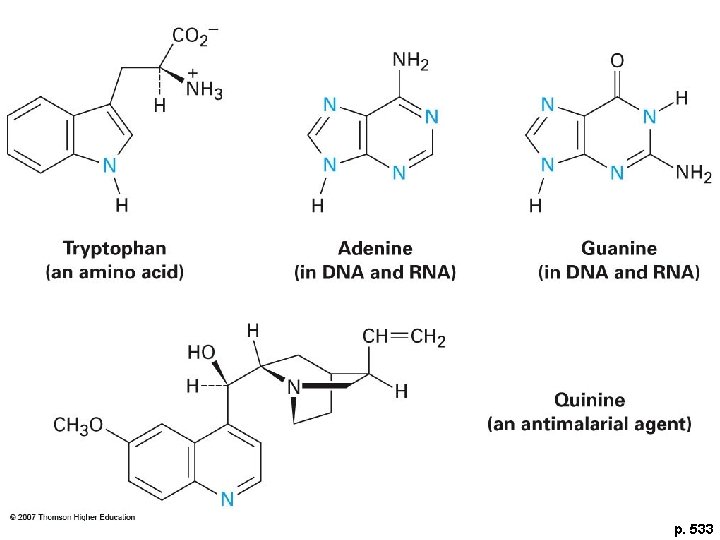
p. 533

End
- Slides: 56