History of Atomic Structure Democritus Aristotle Ancient Philosophy


















- Slides: 20

History of Atomic Structure

Democritus Aristotle

Ancient Philosophy • • Who: Aristotle, Democritus When: More than 2000 years ago Where: Greece What: Aristotle believed in 4 elements: Earth, Air, Fire, and Water. Democritus believed that matter was made of small particles he named “atoms”.

Alchemists • • Who: European Scientists When: 800 – 900 years ago Where: Europe What: Their work developed into what is now modern chemistry. • Why: Trying to change ordinary materials into gold.

Alchemic Symbols

John Dalton

Particle Theory • • Who: John Dalton When: 1808 Where: England What: Described atoms as tiny particles that could not be divided. Thought each element was made of its own kind of atom.

J. J. Thompson

Discovery of Electrons • • Who: J. J. Thompson When: 1897 Where: England What: Thompson discovered that electrons were smaller particles of an atom and were negatively charged. • Why: Thompson knew atoms were neutrally charged, but couldn’t find the positive particle.

Ernest Rutherford

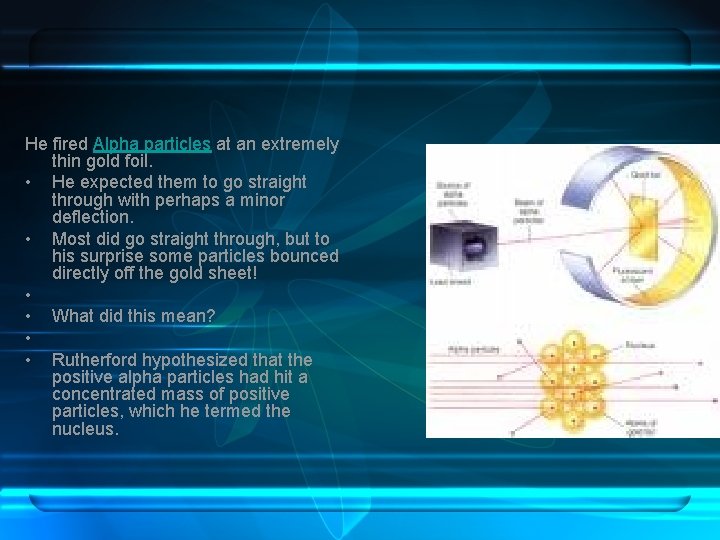
Atomic Structure I • • Who: Ernest Rutherford When: 1911 Where: England What: Conducted an experiment to isolate the positive particles in an atom. Decided that the atoms were mostly empty space, but had a dense central core. • Why: He knew that atoms had positive and negative particles, but could not decide how they were arranged.

He fired Alpha particles at an extremely thin gold foil. • He expected them to go straight through with perhaps a minor deflection. • Most did go straight through, but to his surprise some particles bounced directly off the gold sheet! • • What did this mean? • • Rutherford hypothesized that the positive alpha particles had hit a concentrated mass of positive particles, which he termed the nucleus.


Rutherford’s Lab in Cambridge


Atomic Structure II • • Who: Niels Bohr When: 1913 Where: England What: Proposed that electrons traveled in fixed paths around the nucleus. Scientists still use the Bohr model to show the number of electrons in each orbit around the nucleus. • Why: Bohr was trying to show why the negative electrons were not sucked into the nucleus of the atom.

Electron Cloud Model • Electrons travel around the nucleus in random orbits. • Scientists cannot predict where they will be at any given moment. • Electrons travel so fast, they appear to form a “cloud” around the nucleus.

Electron Cloud Model

Neils Bohr

• Rays or Particles? • Curie-Joliots in Paris, had bombarded beryllium with alpha-particles, and found that very penetrating gamma rays were emitted. Cockcroft and Walton thought that similar rays should be observed when they bombarded light elements with protons. Unfortunately, the Curie-Joliots were mistaken in identifying the 'radiation'. In January 1932 James Chadwick repeated their experiments and found that the reaction produced neutral particles, neutrons, that Rutherford had predicted in 1920. A few months after Chadwick's discovery Rutherford came to Cockcroft and Walton and told them they 'ought to put in a fluorescent screen and get on with the job'. Rutherford was clearly hoping to see alpha-particles, and a fluorescent screen would be the best way to detect them. On 14 April 1932 Walton set up the tube and bombarded lithium with high energy protons. He then crawled into the little observation cabin set up under the apparatus and immediately saw scintillations of the fluorescent screen. The reaction was giving off alpha-particles. • •